Shares of Ocugen, Inc. (OCGN) fell 9.2% on Friday after the biopharmaceutical company disclosed that the U.S. Food and Drug Administration (FDA) issued a clinical hold on its Investigational New Drug application (IND) to evaluate the COVID-19 vaccine candidate, BBV152.
BBV152, also known as COVAXIN, was developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV). Ocugen is co-developing COVAXIN in the U.S. and Canadian markets.
The regulator seeks to identify the shortfalls in the vaccine candidate that led to the clinical hold and provide suggestions on how to address those deficiencies. (See Ocugen stock charts on TipRanks)
Meanwhile, Ocugen expects to receive a formal written response with the additional information from the FDA and plans to work with the FDA to resolve its queries.
See Insiders’ Hot Stocks on TipRanks >>
Analyst’s Recommendation
On Novemeber 8, Noble Financial analyst Robert LeBoyer reiterated a Buy rating on Ocugen with a price target of $15 (120.6% upside potential).
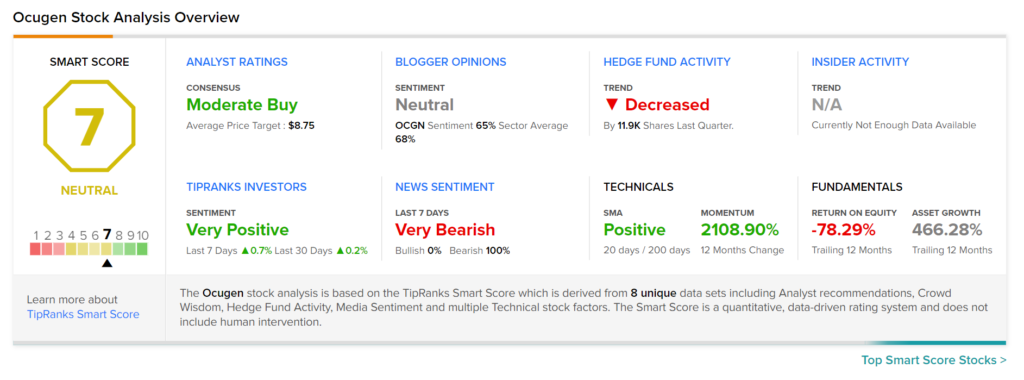
Consensus among analysts is a Moderate Buy based on 2 Buys and 2 Holds. The average Ocugen price target stands at $8.75, which implies upside potential of 28.7% from current levels. Shares of the company have declined 24.7% over the past six months.
Smart Score
According to the TipRanks’ Smart Score system, Ocugen gets a 7 out of 10, which indicates that the stock is likely to perform in line with market averages.
Related News:
Italian Antitrust Regulator Penalizes Google, Apple
Novartis Aims to Make Healthcare Affordable in Sub-Saharan African Region
Micron, United Micro Agree to Withdraw Plaints Over Intellectual Property