The U.S. Food and Drug Administration (FDA) has permitted state-licensed pharmacists to prescribe Pfizer’s (NYSE: PFE) COVID-19 oral antiviral pill Paxlovid to curb rising cases. Shares gained 2.6% on the news and ended the day at $52.75 yesterday.
Don't Miss our Black Friday Offers:
- Unlock your investing potential with TipRanks Premium - Now At 40% OFF!
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
As per a Reuters report, Paxlovid has been approved for use and is freely available as a course of treatment in the U.S. since December 2021. Yet, less than half of the four million courses distributed to pharmacists have been utilized for the treatment.
The Protocol for Prescribing Paxlovid
Paxlovid is an oral drug to be administered within five days of the onset of symptoms from the virus. The drug is effective in mild to moderate cases of COVID-19 and reduces the chances of severity and hospitalization.
According to protocol, patients who wish to avail of the drug must carry their health records to the pharmacists to check for liver and kidney problems. Moreover, the American Medical Association (AMA) has suggested that the prescription should be done by a doctor wherever possible, since Paxlovid is not suitable for everyone and that the patients need to be monitored for side effects and follow-up care.
To that effect, the FDA has suggested that where the health records are not substantial enough to prove the safety of the drugs, pharmacists should refer the patients to a healthcare professional licensed to prescribe drugs. For example, patients with lower kidney function should be administered lower doses of the pill, the FDA said.
Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research, said, “Since Paxlovid must be taken within five days after symptoms begin, authorizing state-licensed pharmacists to prescribe Paxlovid could expand access to timely treatment for some patients.”
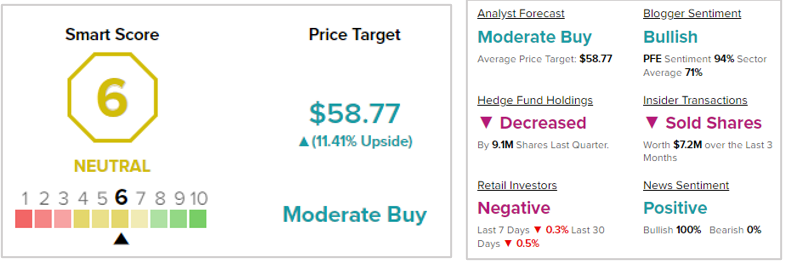
Moderate Buy for PFE
Analysts on the Street are cautiously optimistic about PFE stock with a Moderate Buy consensus rating based on six Buys and eight Holds. The average Pfizer price target of $58.77 implies 11.4% upside potential to current levels. Meanwhile, the stock has lost 5.4% so far this year.
Neutral Rating for PFE
According to TipRanks’ Smart Score, Pfizer has a score of six, indicating that the stock is likely to perform in line with the market. Bloggers and news articles are bullish on the stock.
However, hedge funds have decreased their holdings of PFE stock by 9.1 million shares in the last quarter. Moreover, retail investors have reduced their exposure to PFE stock and corporate insiders have sold PFE stock worth $7.2 million during the last three months.
Ending Thoughts
Currently, Paxlovid is available only under Emergency Use Authorization (EUA). Pfizer is pushing for the pill to receive full approval even to treat high risk COVID-19 patients. If received the pill could generate billions in revenue alongside its COVID-19 vaccine Comirnaty.



















