Eli Lilly and Co. (LLY) announced that the U.S. Food and Drug Administration (FDA) had approved its atopic dermatitis (eczema) drug, EBGLYSS (lebrikizumab-lbkz). This drug will be used for the treatment of adults and children 12 years of age and older who weigh at least 88 pounds (40 kg).
Stay Ahead of the Market:
- Discover outperforming stocks and invest smarter with Top Smart Score Stocks
- Filter, analyze, and streamline your search for investment opportunities using Tipranks' Stock Screener
U.S. FDA Grants Approval to EBGLYSS Based on Various Studies
Furthermore, the FDA granted the approval for EBGLYSS (lebrikizumab-lbkz) based on the ADvocate 1, ADvocate 2, and ADhere studies, which involved over 1,000 participants (aged 12 and older) with moderate-to-severe eczema. Their eczema was unresponsive to topical treatments. As a result, these patients were treated with Ebglyss during the clinical trials and the drug met the primary endpoint at 16 weeks.
Following the approval, EBGLYSS will be available in the U.S. over the coming weeks. The drug has already been approved in Europe and Japan and is expected to expand into more markets this year, with Lilly holding exclusive global rights outside Europe.
Approval of EBGLYSS Strengthens LLY’s Pipeline
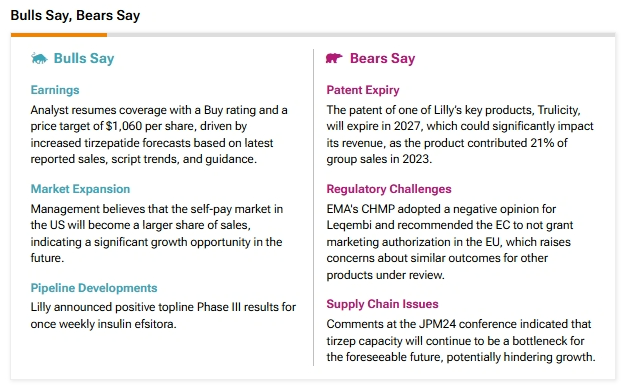
The approval of EBGLYSS is expected to strengthen LLY’s drug pipeline. Furthermore, according to the TipRanks “Bulls Say, Bears Say,” analysts bullish on LLY believe that there is a significant growth opportunity for the pharma company in the U.S. from customers paying by themselves for a drug. As a result, analysts see that the self-pay market in the U.S. will become a “larger share of sales.”
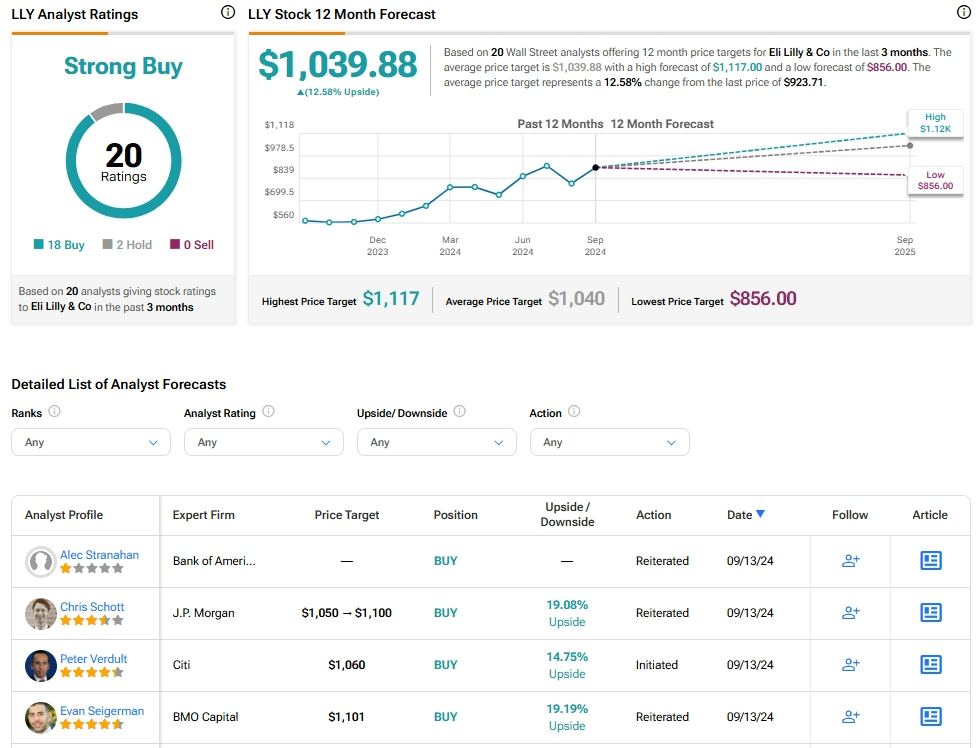
Is Eli Lilly a Buy, Sell, or Hold?
Analysts remain bullish about LLY stock, with a Strong Buy consensus rating based on 18 Buys and two Holds. Year-to-date, LLY has surged by more than 90%, and the average LLY price target of $1,039.88 implies an upside potential of 12.6% from current levels.