Evoke Pharma (EVOK) shares leaped 86% in pre-market trading after the U.S. Food and Drug Administration approved its Gimoti nasal spray for the treatment of acute and recurrent diabetic gastroparesis.
The stock surged to $4.38 in early U.S. trading on Monday. The New Drug Application (NDA) status granted by the FDA will allow Evoke to start with the commercialization of its Gimoti nasal spray in the U.S. with sales expected to be initiated in the fourth quarter.
“This approval represents the first novel pharmaceutical treatment for gastroparesis in several decades. We are excited to be able to offer health care providers and their patients a unique non-oral treatment option to relieve symptoms,” said Evoke President and CEO David Gonyer. “Together with our partner Eversana, we are now fully focused on executing our commercialization strategy for Gimoti by leveraging Eversana’s integrated suite of capabilities and highly experienced sales and marketing team.”
The FDA milestone gives Evoke access to its $5 million credit line from Eversana to support manufacturing and commercialization of the nasal spray. As of May 31, the pharmaceutical company had cash and cash equivalents of about $4.7 million.
The cash and cash equivalents, together with the Eversana credit, will be enough to support its operations into 2021, the company said.
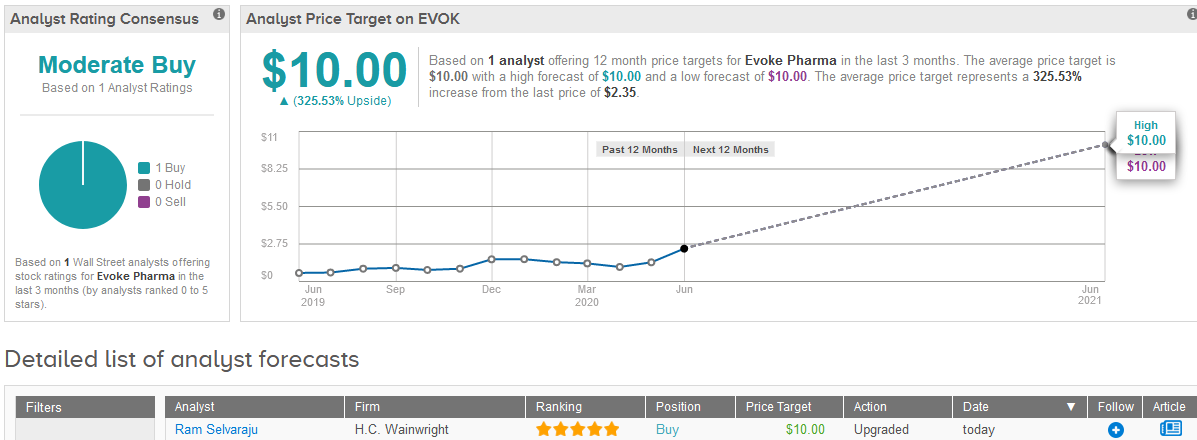
Following the announcement, five-star analyst Ram Selvaraju at H.C. Wainwright raised the stock’s rating to Buy from Hold with a $10 price target, reflecting a $325% upside potential in the shares in the coming 12 months. Selvaraju’s bullish outlook is driven by the forecast that peak U.S. annual sales will amount to >$300 million by 2027.
Looking to 2021, the analyst expects Gimoti to generate $14 million in sales, which translates into about $13 million in revenue for Evoke.
“Assuming this modestly-paced trajectory for Gimoti, we anticipate that Evoke could achieve cash flow breakeven in 1H22,” Selvaraju wrote in a note to investors.
Related News:
Sanofi, Regeneron Win China Approval For Dupixent Dermatitis Drug
Regeneron Starts Human Clinical Trials Of Covid-19 Antibody Cocktail
Emergent Bio Signs Covid-19 Vaccine Manufacturing Deal With AstraZeneca