Pfizer Inc. (PFE) and BioNTech SE’s (BNTX) COVID-19 vaccine received approval from the European Union’s regulatory authority for children between the ages of 5 and 11.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The approval will in aid the mitigation of the accelerated rise in COVID-19 cases in Europe. Shares closed at $50.89 on November 25.
According to Reuters, the European Medicines Agency (EMA) has approved the vaccine, Comirnaty, as an injection (in the upper arm) in two 10 microgram doses, to be administered three weeks apart .
In a clinical trial of children between the ages of 5-11 years, Pfizer and BioNTech said that their vaccine demonstrated 90.7% efficacy against the coronavirus.
As per the study, the side effects of the vaccine were similar to those observed in people of ages 12 and above. The side effects are usually mild or moderate and go away within a few days of receiving the shot. These include pain at the injection site, tiredness, headache, redness and swelling at the site of the injection, muscle pain, and chills.
Commenting on the approval, the EMA said, “The benefits of Comirnaty in children aged 5 to 11 outweigh the risks, particularly in those with conditions that increase the risk of severe COVID-19.”
The first low-dose shots of the vaccine will be delivered to the EU on December 20, 2021. The final approval for the vaccine will be given by the European Commission, which typically follows EMA recommendations, and the decision is expected to come on Friday.
Comirnaty has already received approval for use in children aged between 12-17 years across 27 EU countries since May 2021, and in adults since December 2020. Adults are given a 30 microgram dose.
Other countries which have already approved vaccines for the ages 5 to 11 and younger include the United States, Canada, Israel, China, and Saudi Arabia.
See Analysts’ Top Stocks on TipRanks >>
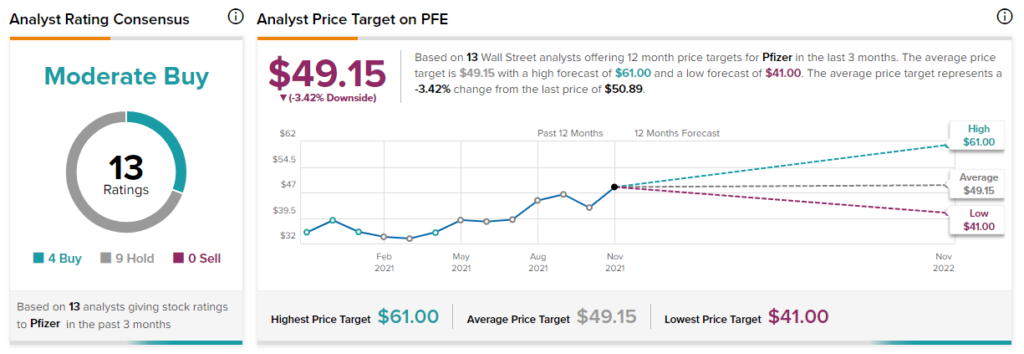
Target Price
The Wall Street community is cautiously optimistic about the stock with a Moderate Buy consensus rating based on 4 Buys and 9 Holds. The average Pfizer price target of $49.15 implies downside potential of 3.4% to current levels. Shares have gained 36.7% over the past year.
Related News:
Deere Posts Stellar Q4 Results; Shares Up 5%
Blink Opens India Office to Expand International Footprint
Novavax Files for Interim Authorization of COVID-19 Vaccine in Singapore









