Shares of Eiger Biopharmaceuticals popped more than 12% in Friday’s extended market session after the US Food and Drug Administration (FDA) approved its Zokinvy (lonafarnib) capsules to reduce the risk of death in young patients suffering from Hutchinson-Gilford progeria syndrome (HGPS).
Stay Ahead of the Market:
- Discover outperforming stocks and invest smarter with Top Smart Score Stocks
- Filter, analyze, and streamline your search for investment opportunities using Tipranks' Stock Screener
Eiger (EIGR) said that the FDA approval of Zokinvy is also for the treatment of certain processing-deficient progeroid laminopathies in patients one year of age and older. Zokinvy is not approved for use in patients with other progeroid syndromes or laminopathies.
Progeria and progeroid laminopathies are separate and ultra-rare, genetic, premature aging diseases that advance death in young patients. Disease symptoms include growth failure, loss of body fat and hair, aged-looking skin, stiffness of joints, hip dislocation, generalized atherosclerosis, cardiovascular disease and stroke. Untreated children with progeria die of heart disease at an average age of 14.5 years. There are 20 children and young adults with Progeria and PL identified and followed in the US.
Zokinvy, a farnesyltransferase inhibitor, is an oral medication that helps prevent the buildup of defective progerin or progerin-like protein.
“With today’s approval, Zokinvy is the first FDA-approved medication for these devastating diseases. The FDA will continue to work with stakeholders to advance the development of additional new, effective and safe therapies for these patients,” said FDA’s Hylton V. Joffe.
The effectiveness of Zokinvy for the treatment of Hutchinson-Gilford progeria syndrome was based on the study of 62 patients from two single-arm trials that were compared to matched, untreated patients from a separate natural history study. In patients with progeria, Zokinvy reduced the incidence of mortality by 60% and increased average survival time by 2.5 years.
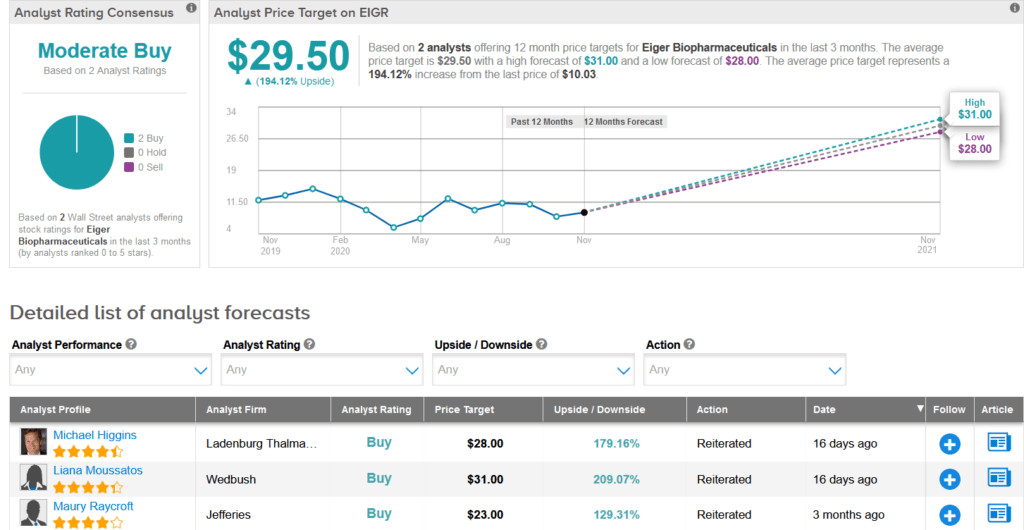
Earlier this month, Ladenburg Thalmann analyst Michael Higgins maintained a Buy rating on the stock with a 28% price target. This target suggests shares could climb 179% higher over the coming year.
“As a reminder, lonafarnib was granted Orphan Drug Designation and Breakthrough Therapy Designation, and Eiger also earned a Rare Pediatric Disease Designation which allows for a transferable priority review voucher (PRV) upon approval, which we value at ~$100M (to be split 50/50 with the Progeria Research Foundation) based on recent transactions,” Higgins wrote in a note to investors. “We are expecting approval and launch in the EU in 1H’21. We expect the cash of ~$125M to last through this approval and launch.” (See EIGR stock analysis on TipRanks)
All in all, the other analyst recently covering EIGR echoes Higgins’ sentiment with a Buy rating, which together add up to a Moderate Buy consensus. With shares down 32% so far this year, the average forecast of $29.50, puts the upside potential at a whopping 194%.
Related News:
FDA To Review Pfizer-BioNTech Covid-19 Vaccine On Dec. 10
Pfizer Expected to File FDA EUA Application for COVID-19 Vaccine Today
Mesoblast Teams Up With Novartis to Develop COVID-19/ARDS Stem Cell Therapy; Shares Up 17%



















