According to a July 24 filing, the US Food & Drug Administration (FDA) has now approved Dr Reddy’s Laboratories’ (RDY) Xeglyze (abametapir) for the treatment of head lice.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Xeglyze is a topical treatment of head lice infestation for patients 6 months of age and older that involves just a single application of the product.
“Despite its prevalence and high cost to the community, there have been few major advances in controlling head lice infestation in recent years. Most pediculicide products have little ovicidal activity and require two treatments… Non-compliance with this regimen and the difficulty in choosing the optimal time for the second application, are major drawbacks” commented Dr Reddy’s.
In contrast “Xeglyze, a topical formulation containing abametapir; an inhibitor of metalloproteases, has demonstrated both ovicidal and lousicidal activity and offers the potential for a more effective treatment using only a single application.”
Two identical multi-center, randomized, double-blind, vehicle-controlled trials were conducted in 704 patients 6 months of age and older with head lice infestation. All patients received a single application of either the treatment or vehicle control. Efficacy was assessed as the proportion of index subjects who were treated with a single 10 minute application and were free of live lice at all follow-up visits on Days 1, 7, and 14.
For both the first and second trial Xeglyze delivered efficacy of over 80%, versus the control at 50.9% in the first trial and 47.2% in the second trial.
Dr Reddy’s snapped up the exclusive rights to Xeglyze in North America, India, Russia and the CIS, Australia, New Zealand and Venezuela from Hatchtech Pty Ltd, an Australian pharmaceutical, back in 2015.
As part of the agreement, Dr. Reddy’s agreed to pay Hatchtech an $10M upfront and up to $50M million based on pre commercialization milestones and an undisclosed amount based on post commercialization milestones, linked to achievement of annual net sales targets.
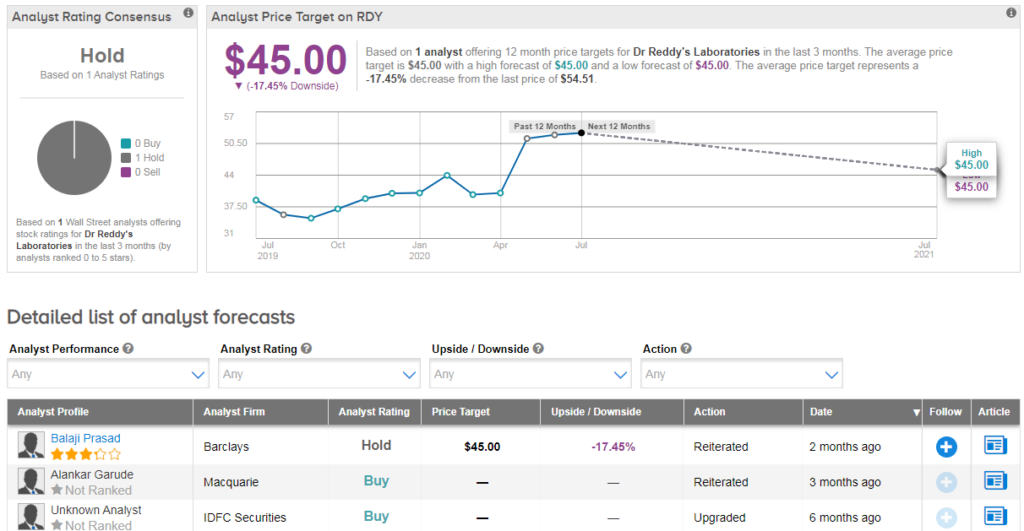
Shares in Dr Reddy’s are now up 34% year-to-date, but the stock shows a Hold analyst consensus on TipRanks. (See RDY stock analysis on TipRanks).
Related News:
Gilead’s Kite Gets FDA Nod For Tecartus Blood Cancer Treatment
NuVasive Spikes 5% After-Hours On Sharp Procedure Rebound
Acadia Plunges 12% As Depressive Study Misses Goals; Analyst Says Buy









