Shares in Sorrento (SRNE) are rallying 17% in Thursday’s pre-market trading after the company announced that an Application for Emergency Use Authorization (EUA) is under review at the US FDA for is Covid-19 diagnostic test kit.
Its COVI-TRACK in vitro diagnostic test kit enables the independent detection of IgG and IgM antibodies in sera of patients exposed to the SARS-CoV-2 virus.
The rapid antibody test allows for results to be available in eight minutes or less. It reveals whether someone has been exposed to or potentially had coronavirus, and subsequently created antibodies to combat the infection.
Analytical validation was performed by testing sample cohorts from healthy donors and confirmed positive COVID-19 patient samples by RT-PCR testing, and the assay demonstrated a specificity greater than 97% and diagnostic sensitivity of greater than 94%.
Upon issuance of an EUA, the COVI-TRACK test will be available for distribution to clinical testing sites nationwide, says Sorrento.
The company has already secured manufacturing capacity to support the production of up to five million test kits per month.
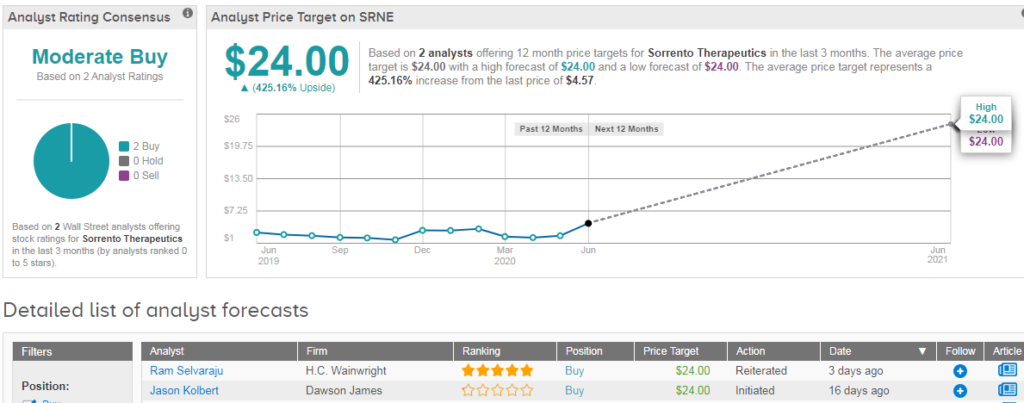
Shares in Sorrento are currently trading up 35% on a year-to-date basis, and analysts have a Moderate Buy consensus on the stock’s outlook. The $24 average analyst price target indicates significant upside potential from current levels. (See SRNE stock analysis on TipRanks).
Last week, Sorrento disclosed that in preclinical trials, the company’s monoclonal antibody (MaB), STI-4938, code named COVIDTRAP, inhibited SARS-CoV-2 viral infection in vitro.
Sorrento has another antibody in development, STI-1499 (COVI-SHIELD), which also recently demonstrated promising in vitro results as it was able to completely block the SARS-CoV-2 virus.
While it is still early on for both antibodies, H.C. Wainwright analyst Ram Selvaraju is encouraged by the additional positive results. “Together with Sorrento’s previously announced preclinical neutralizing antibody candidate, STI-1499, COVIDTRAP may provide a potent antidote against COVID-19,” the analyst said.
Related News:
5 Promising Covid-19 Vaccines Picked For Trump’s Operation Warp Speed
What Would a Merger Mean for Gilead? Top Analyst Weighs In
Soleno Plunging 48% In Pre-Market On Obesity Study Failure
















