Sanofi (SNYNF) and Regeneron Pharma (REGN) have announced that the US Phase 3 trial of Kevzara (sarilumab) 400 mg in COVID-19 patients requiring mechanical ventilation did not meet its primary and key secondary endpoints when Kevzara was added to best supportive care compared to placebo.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Minor positive trends were observed- but these trends did not reach statistical significance and were countered by negative trends in a subgroup of critical patients who were not mechanically ventilated at baseline.
In the primary analysis group, adverse events were experienced by 80% of Kevzara patients and 77% of placebo patients. Serious adverse events that occurred in at least 3% of patients and more frequently among Kevzara patients were multi organ dysfunction syndrome (6% Kevzara, 5% placebo) and hypotension (4% Kevzara, 3% placebo).
Based on the results, the US trial has now been stopped, including in a second cohort of patients who received a higher dose of Kevzara (800 mg). Detailed results will be submitted to a peer-reviewed publication later this year.
The primary analysis group included 194 patients who were critically ill with COVID-19 and receiving mechanical ventilation at the time of enrolment.
The Kevzara trial was designed after a small (n=21), single-arm study in China among mostly severe, febrile hospitalized COVID-19 patients found elevated IL-6 levels and suggested that inhibiting this pathway with the IL-6 blocker tocilizumab rapidly reduced fever and improved oxygenation in severe patients, allowing for successful hospital discharge.
A separate Sanofi-led trial outside of the U.S. in hospitalized patients with severe and critical COVID-19 using a different dosing regimen is ongoing.
Kevzara is currently approved in multiple countries to treat adults with moderately to severely active rheumatoid arthritis who have not responded to or tolerated previous therapy.
Following the news Nasdaq-listed shares in Sanofi fell 3% after-hours, while Regeneron pulled back 2%. Nonetheless, Regeneron is still trading up an impressive 65% year-to-date.
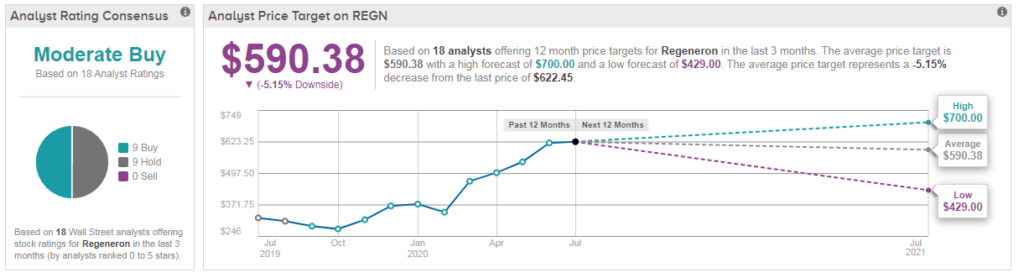
In light of Regeneron’s recent stock advance, the $590 average analyst price target now implies 5% downside potential in the coming 12 months. Overall, the 18 analysts covering the stock are divided between 9 Buy and 9 Hold ratings adding up to a Moderate Buy consensus. (See Regeneron stock analysis on TipRanks)
Related News:
Moderna Sinks 5% On Covid-19 Vaccine Delay Reports
Gilead To Acquire Stake in Cancer Drug Developer Pionyr For $275 Million
Merck, BioInvent Enroll First Patient In Solid Tumor Combo Trial









