French drugmaker Sanofi (SNYNF) and GlaxoSmithKline (GSK) will be granted up to $2.1 billion by the U.S. government for the development of their coronavirus vaccine candidate, including clinical trials, as well, for manufacturing scale-up and delivery of an initial 100 million doses.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Under the terms of the collaborative effort, Sanofi will receive the majority of the U.S. government funding. Moreover, the U.S. government will have an option to purchase an additional 500 million doses. The vaccine candidate, developed by Sanofi in partnership with GSK, is based on the recombinant protein-based technology used by Sanofi to produce an influenza vaccine, and GSK’s established pandemic adjuvant technology.
“The global need for a vaccine to help prevent COVID-19 is massive, and no single vaccine or company will be able to meet the global demand alone,” said Thomas Triomphe, Global Head of Sanofi Pasteur.
Part of the agreement includes collaboration with the U.S. Department of Health and Human Services (HHS) and Department of Defense, which the companies expect will help fund vaccine development activities and secure scale-up manufacturing capabilities in the US, resulting in a significant increase in capacity.
The Sanofi-GSK funding is provided by Operation Warp Speed (OWS), the U.S. government program to accelerate the development, manufacturing, and distribution of COVID-19 vaccines available for Americans by Jan. 2021.
The two companies said they expected their vaccine candidate to enter Phase 1/2 clinical trials in September, followed by a Phase 3 study by the end of 2020. If the data are positive, the companies can request U.S. regulatory approval in the first half of 2021. In parallel, Sanofi and GSK are scaling up manufacturing of the antigen and adjuvant to produce up to one billion doses per year globally.
Separately Sanofi and GSK announced that they are in advanced discussions, with the European Commission (EC) for the supply of up to 300 million doses of their COVID-19 vaccine candidate. The doses would be manufactured in European countries including France, Belgium, Germany and Italy.
Shares in Sanofi have recovered since reaching a low in March and are now trading about 5.5% higher than at the beginning of the year.
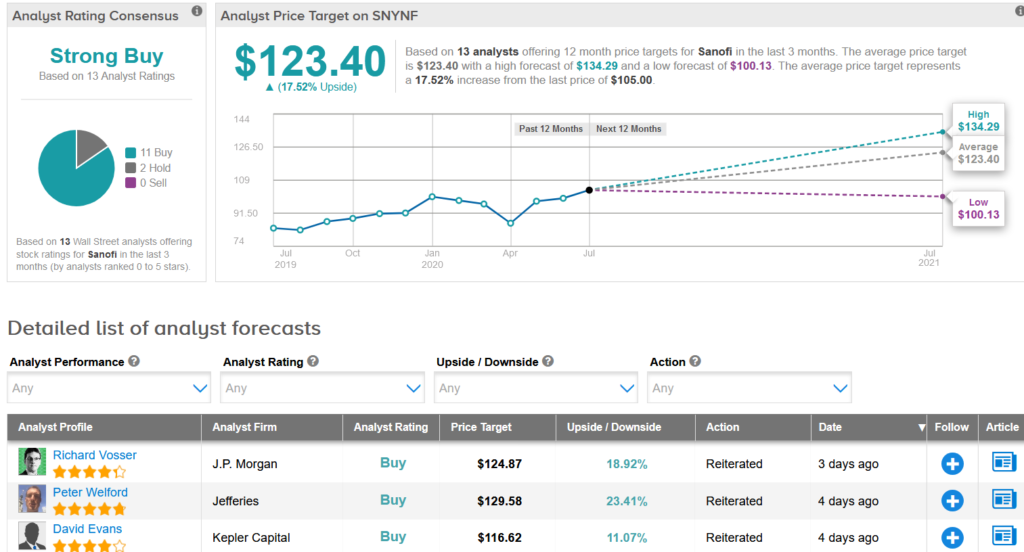
TipRanks data shows that the majority of analysts have a bullish outlook on the stock. The Strong Buy consensus boasts 11 Buy ratings versus 2 Hold ratings. The $123.40 average price target reflects 18% upside potential in the shares in the coming 12 months. (See Sanofi stock analysis on TipRanks)
Related News:
Sanofi, GSK Agree UK Supply Deal For 60M Covid-19 Vaccine Doses
Pfizer, BioNTech Rise As Phase 2/3 Covid-19 Vaccine Trial Kicks Off
AstraZeneca To Pay Up To $6B For Daiichi Cancer Drug Deal









