Sanofi (SNYNF) and GSK (GSK) have now confirmed an agreement with the UK government for the supply of up to 60 million doses of a COVID-19 vaccine. The agreement is still subject to final contract, says Sanofi.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The vaccine candidate, developed by Sanofi in partnership with GSK, is based on the recombinant protein-based technology used by Sanofi to produce an influenza vaccine, and GSK’s established pandemic adjuvant technology.
“With our partner GSK, we are pleased to cooperate with the UK government as well as several other countries and global organizations as part of our ongoing efforts to develop a safe and effective vaccine and make it available as quickly as possible. We greatly appreciate the UK government’s support of this shared vision,” said Thomas Triomphe of Sanofi Pasteur.
Sanofi is leading the clinical development and registration of the COVID-19 vaccine and expects a Phase 1/2 study to start in September, followed by a Phase 3 study by the end of 2020. If the data is positive, regulatory approval could be achieved by the first half of 2021. In parallel, Sanofi and GSK are scaling up manufacturing of the antigen and adjuvant to produce up to one billion doses per year overall.
Active discussions on supply of the vaccine are ongoing with global organizations, the U.S. and the EU Commission, with France and Italy on the negotiation team, says Sanofi.
Separately, Sanofi is also developing a messenger RNA vaccine candidate in partnership with Translate Bio (TBIO). With several innovative vaccine platforms currently being investigated across the industry, mRNA is considered among the most promising, the company says.
It expects a Phase 1 study to start by the end of the year, and, if the data is positive, an approval at the earliest in the second half of 2021. Translate Bio has established mRNA manufacturing capacity and Sanofi expects to be able to supply annual capacity of 90 to 360 million doses.
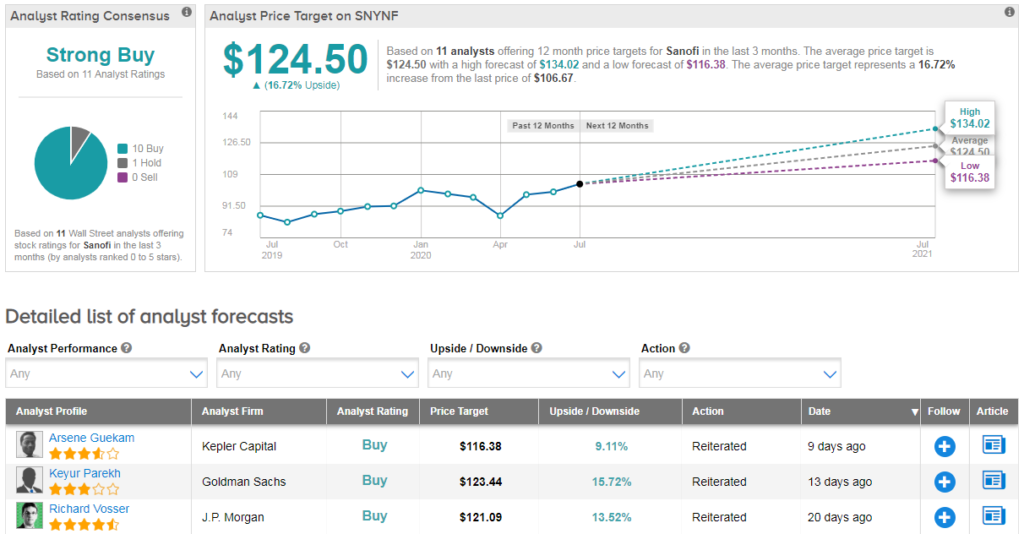
Sanofi scores a bullish Strong Buy Street consensus, with an average analyst price target of $125 (17% upside potential). The company has just announced an earnings beat with Q2 Non-GAAP EPS of €1.28 beating Street estimates by €0.06, however revenue of €8.21B fell 5% year-over-year and fell short of consensus expectations by €50M. (See Sanofi stock analysis on TipRanks).
Related News:
Pfizer, BioNTech Rise As Phase 2/3 Covid-19 Vaccine Trial Kicks Off
AstraZeneca To Pay Up To $6B For Daiichi Cancer Drug Deal
Moderna Could Charge $50-$60 Per Covid-19 Vaccine Course- Report









