Qiagen announced the rollout of its new access anti-SARS-CoV-2 antibody test, an easy-to-use digital test, to be shipped to the US as early as this month.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Qiagen (QGEN) said that the test which is done on a portable device and provides results in about 10 minutes can detect antibodies in people exposed to the SARS-CoV-2 virus. The antibody test promises to be a a cost-effective way to detect immune responses in people who have been exposed to the virus.
The launch of the test, which was developed in partnership with the Australian digital diagnostics company Ellume, comes after its submission by Qiagen to the US Food and Drug Administration (FDA) for Emergency Use Authorization (EUA). First shipments are planned for late August 2020.
“Increased testing is the only way to gain visibility on the magnitude of the pandemic, which will ultimately lead to helping control it,” said Davide Manissero, Qiagen’s Chief Medical Officer. “As a trusted partner in the fight against COVID-19, QIAGEN has now added the Access Anti-SARS-CoV-2 to our portfolio as a smart solution for antibody testing that provides results with confidence. This is a rugged and portable platform that requires no hardware, can process a wide range of tests and provides fast results.”
The test is performed on the eHub, a small portable digital device that provides results in 10 minutes. The eHub device can handle up to eight patient samples simultaneously and can perform up to 32 total tests per hour. The nanoparticle fluorescent detection technology uses serum or plasma from patient samples.
The serological test has been shown to have 100% sensitivity and 100% specificity, Qiagen said. The company added that serological testing for antibodies is central to identifying people who have been recently infected by the virus or have been infected in the recent past, especially those who did not show any symptoms.
A CE-IVD marking approval for Europe and other markets is planned in the coming weeks.
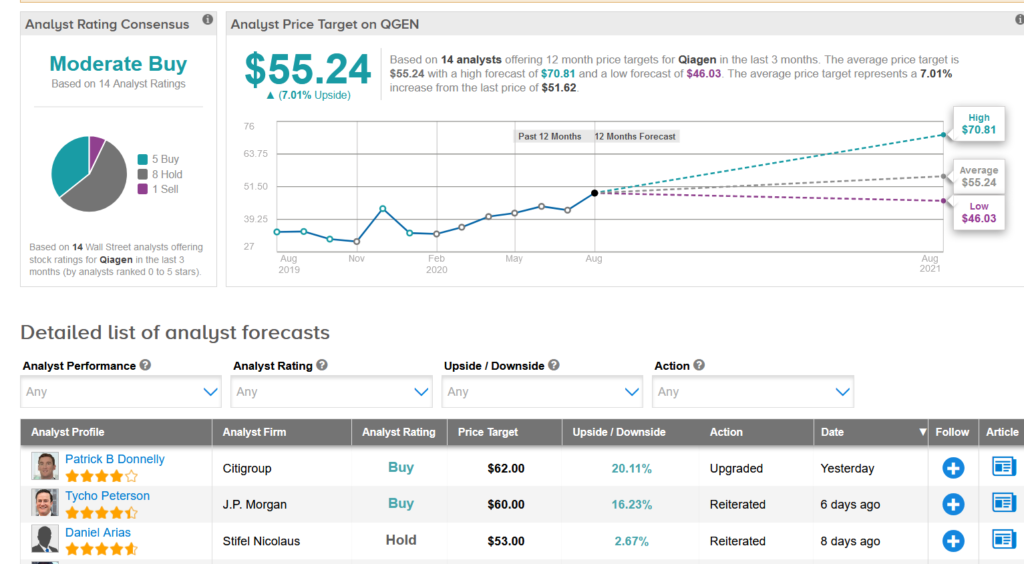
Qiagen shares have ballooned 53% so far this month with the average of analysts cautiously optimistic on the stock with a Moderate Buy consensus. Furthermore, the $55.24 average price target implies another 7% upside potential is lying ahead in the coming year.
In a bullish note, Citigroup analyst Patrick B Donnelly this week upgraded QGEN to Buy from Neutral and ramped up the price target to $62 (20% upside potential) from $48, saying that the stock’s current valuation does not fully reflect the upside drivers related to Covid-19 testing and better management execution in the underlying business. (See QGEN stock analysis on TipRanks)
Donnelly notes that better visibility into the second half of 2020 volumes and related capacity ramps have boosted confidence in the durability of Qiagen’s Covid-19 testing headwinds, which are expected to drive continued demand for the company’s sample prep solutions and other products tied to coronavirus testing.
The analyst added that he views the company’s 2020 and 2021 guidance as conservative.
Related News:
Lowe’s Hikes Quarterly Cash Dividend By 9% After 2Q Surprises Investors
AstraZeneca Rises On Report Trump Could Fast-Track Covid-19 Vaccine Candidate
Arcturus Inks Deal with Israel For Covid-19 Candidate; Analyst Sees 25% Upside