Pfizer is planning to file an application to the U.S. Food and Drug Administration (FDA) for approval of its COVID-19 vaccine candidate in late November, its CEO Albert Bourla said. The stock closed 3.8% higher on Friday.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Initially Pfizer (PFE), which is developing the mRNA-based vaccine candidate against SARS-CoV2 together with BioNTech, said that it seeks to establish whether or not the vaccine is effective by the end of October. This will involve data, which will need to demonstrate that the vaccine can help prevent COVID-19 disease in at least a majority of vaccinated patients.
Second and equally important, the vaccine must be proven safe, with robust safety data generated from thousands of patients.
“So let me be clear, assuming positive data, Pfizer will apply for Emergency Authorization Use in the U.S. soon after the safety milestone is achieved in the third week of November,” Bourla said in an open letter. “All the data contained in our U.S. application would be reviewed not only by the FDA’s own scientists but also by an external panel of independent experts at a publicly held meeting convened by the agency.”
And finally, Pfizer must demonstrate that the vaccine can be consistently manufactured at the highest quality standards, Bourla added. Previously, the two companies had said that, if regulatory approval is obtained, they would be seeking to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses by the end of 2021.
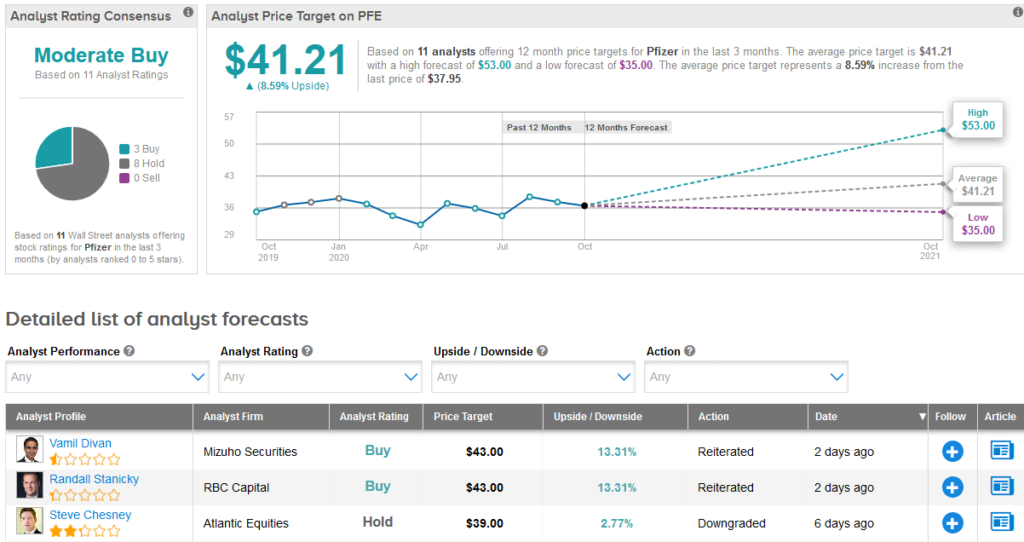
Shares in Pfizer are down 3.1% year-to-date, but the analyst consensus currently has a cautiously optimistic Moderate Buy on the stock. That’s based on 3 Buys and 8 Holds over the last three months, with an average price target of $41.21 (8.6% upside potential).
Following Pfizer’s timeline update, Mizuho analyst Vamil Divan reiterated a Buy rating on the stock with a $43 price target.
“Overall things appear very much on track and the speed in potentially getting the vaccine to market is much faster than we would have expected when the pandemic started,” Divan wrote in a note to investors. “We currently estimate sales of $2B in COVID-19 vaccine sales for Pfizer in 2020-2021 combined, before expecting sales to taper off and plateau at $550-600M annually from 2024 and beyond.” (See Pfizer stock analysis on TipRanks)
The analyst added that, “if the vaccine does obtain regulatory approval, the company already has commitments from governments in various developed countries to supply >450M doses at a price that we believe is not less than the $19.50/dose that was agreed to with the U.S. government. That would suggest COVID-19 vaccine sales for Pfizer should actually exceed $8.5B between 2020-2021.”
Related News:
Vertex Sinks 12% On Halt of VX-814 Development; Merrill Lynch Says Buy
J&J Halts Covid-19 Vaccine Trial Due To ‘Unexplained Illness’
Amarin Pops 4% After-Hours On Positive PCI Data For Vascepa