Late-stage biotech Novavax (NVAX) has announced the beginning of a Phase 2b clinical trial in South Africa to evaluate the efficacy of NVX-CoV2373, Novavax’ COVID-19 vaccine candidate.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
NVX‑CoV2373 is a stable, prefusion protein made using the company’s nanoparticle technology and Matrix‑M adjuvant, which is designed to enhance the immune response and stimulate high levels of neutralizing antibodies.
Dr. Shabir Madhi, Professor of Vaccinology at Wits University, will lead the clinical trial, which is supported in part by a $15 million grant from the Bill & Melinda Gates Foundation.
“Because South Africa is experiencing a winter surge of COVID-19 disease, this important Phase 2b clinical trial has the potential to provide an early indication of efficacy, along with additional safety and immunogenicity data for NVX-CoV2373,” said Gregory M. Glenn of Novavax.
The randomized, observer-blinded, placebo-controlled Phase 2b clinical trial of NVX-CoV2373 will include two cohorts. One cohort will evaluate efficacy, safety and immunogenicity in approximately 2,665 healthy adults.
Meanwhile the second cohort will evaluate safety and immunogenicity in approximately 240 medically stable, HIV-positive adults. This allows for evaluation of the vaccine across a diverse, representative study population, the company says.
Novavax expects that, if approved in South Africa, its COVID-19 vaccine would ultimately be supplied to South Africa through Novavax’ recently announced collaboration with the Serum Institute of India.
“The major motivation for the COVID-19 vaccines being evaluated at an early stage in South Africa is to generate evidence in the African context on how well these vaccines work in settings such as our own,” said Shabir Madhi.
In the Phase 1 portion of the Phase 1/2 clinical trial, conducted in Australia, NVX-CoV2373 was generally well-tolerated and elicited robust antibody responses numerically superior to that seen in human convalescent sera.
Phase 2 clinical trials will begin in August. Novavax has secured $2 billion in funding for its global coronavirus vaccine program, including up to $388 million in funding from the Coalition for Epidemic Preparedness Innovations (CEPI).
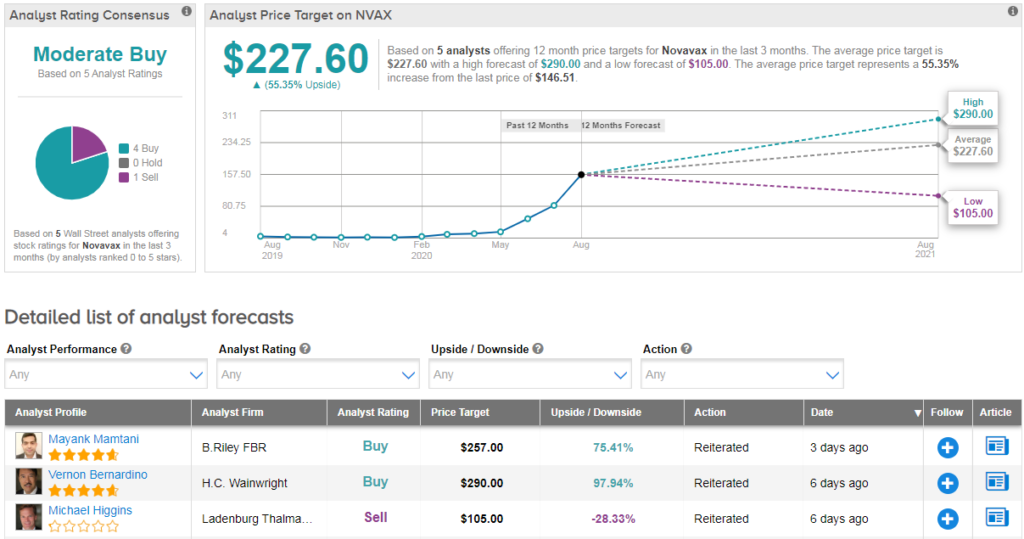
In the run-up to developing a coronavirus vaccine candidate, the stock has exploded by over 3,580%. B.Riley FBR analyst Mayank Mamtani recently reiterated a Buy rating on the stock with a $257 price target (75% upside potential).
“We anticipate NVAX continuing to secure supply agreements to secure region specific demand, totaling in ~2-3 billion ‘2373 doses delivered in 2021-22 timeframe,” Mamtani told investors.
“Management noted for this distribution roadmap to become clearer in coming weeks, which we anticipate to then transition over to purchase agreement contracts with possibly disclosed terms on economics flowing to NVAX and ‘2373 pricing” he added. (See Novavax stock analysis on TipRanks).
The rest of the Street has a cautiously optimistic outlook on the stock. The Moderate Buy analyst consensus shows 4 Buy ratings versus 1 Sell rating. Despite the elevated prices, the $227.60 average analyst price target indicates that shares can rise a further 55%.
Related News:
Masimo Gets FDA Clearance For Tracking Tool In Ventilated Adult Patients
Novavax Pops 7% On South Korea Deal For Global Covid-19 Vaccine Supply
Inovio: Valuation and Volatility Keep This Analyst Sidelined









