Shares in Moderna Inc. (MRNA) shot up 21% after the biotech company reported “positive” interim clinical data for its experimental coronavirus vaccine.
The stock soared 21% to $80.47 in early morning U.S. trading. The clinical stage biotech company said that the Phase 1 study of its novel coronavirus vaccine candidate (mRNA-1273) produced antibodies that would be able to “neutralize” the virus in patients. In addition, different doses of the vaccine given to patients resulted in an increase in immunogenicity, while also triggering an immune response in the body.
“These interim Phase 1 data, while early, demonstrate that vaccination with mRNA-1273 elicits an immune response of the magnitude caused by natural infection,” said Tal Zaks, Chief Medical Officer at Moderna. “When combined with the success in preventing viral replication in the lungs of a pre-clinical challenge model at a dose that elicited similar levels of neutralizing antibodies, these data substantiate our belief that mRNA-1273 has the potential to prevent Covid-19 disease and advance our ability to select a dose for pivotal trials.”
Moderna said that mRNA-1273 was generally found to be safe and well tolerated, with a safety profile consistent with that seen in prior Moderna infectious disease vaccine clinical studies. The company expects the Phase 3 trial to start in July, subject to the finalization of the clinical trial protocol.
“The Moderna team continues to focus on moving as fast as safely possible to start our pivotal Phase 3 study in July and, if successful, file a BLA,” said Stéphane Bancel, CEO at Moderna. “We are investing to scale up manufacturing so we can maximize the number of doses we can produce to help protect as many people as we can from SARS-CoV-2.”
The interim clinical data of the mRNA-1273 Phase 1 study was led by the National Institute of Allergy and Infectious Diseases (NIAID), which is part of the National Institutes of Health (NIH).
Last week, Moderna announced that the mRNA-1273 vaccine candidate was granted fast track status by the U.S. Food and Drug Administration (FDA). The fast track designation is awarded to speed up the regulatory review of the vaccine candidate.
Since the start of the year, the value of Moderna’s shares has quadrupled.
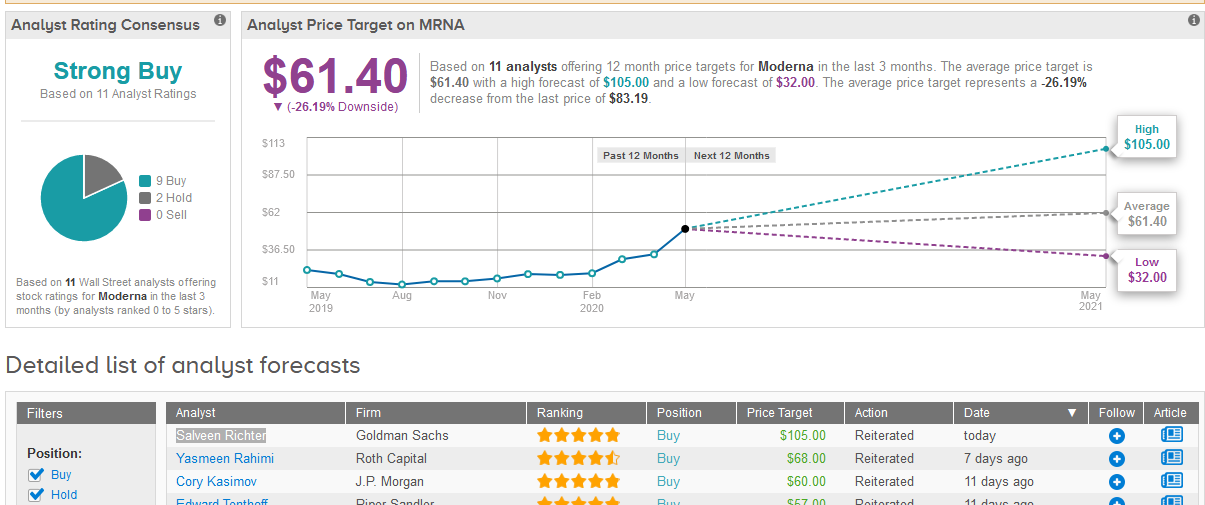
Following the report, five-star analyst Salveen Richter at Goldman Sachs raised Moderna’s price target to $105 from $63 and maintained a Buy rating on the shares.
Although analysts have a Strong Buy consensus rating on Moderna stock based on 9 Buys and 2 Holds, its recent rally means that the $61.40 average price target now indicates 26% downside potential from current levels. (See Moderna stock analysis on TipRanks).
Last week, Yasmeen Rahimi, analyst at Roth Capital said that with the near-term start of a Phase 2 study for its Covid-19 vaccine, the company remains the front-runner in getting a vaccine to pivotal trials with potential approval by 2021. Rahimi has a Buy rating on the stock with a $68 price target.
Related News:
Europe Could Conditionally Approve Gilead’s Remdesivir In Next Few Days
Gilead Signs Remdesivir Licensing Agreements With Five Drugmakers
AstraZeneca Aiming For 30M UK Covid-19 Vaccine Doses By September