Moderna (MRNA) soared a further 16% in Tuesday’s after-market trading after the biotech announced the publication of an interim analysis of the open-label Phase 1 study of mRNA-1273, its vaccine candidate against COVID-19.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Results reaffirmed positive interim data assessment announced on May 18, said Moderna, and show mRNA-1273 induced rapid and strong immune responses against SARS-CoV-2. Neutralizing antibodies were observed in 100% of evaluated participants at the 100 µg dose level selected for the upcoming Phase 3 trial.
Published in The New England Journal of Medicine, the interim analysis evaluated a two-dose vaccination schedule of mRNA-1273 given 28 days apart across three dose levels (25, 100, 250 µg) in 45 healthy adult participants ages 18-55 years, and reported results through Day 57.
The study was led by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
“These Phase 1 data demonstrate that vaccination with mRNA-1273 elicits a robust immune response across all dose levels and clearly support the choice of 100 µg in a prime and boost regimen as the optimal dose for the Phase 3 study,” said Tal Zaks CMO of Moderna. “We look forward to beginning our Phase 3 study of mRNA-1273 this month to demonstrate our vaccine’s ability to significantly reduce the risk of COVID-19 disease.”
Encouragingly, mRNA-1273 was generally safe and well-tolerated, with no serious adverse events reported through Day 57. Adverse events (AEs) were generally transient and mild to moderate in severity.
mRNA-1273 induced binding antibodies to the full-length SARS-CoV-2 Spike protein (S) in all participants after the first vaccination, with all participants seroconverting by Day 15. This is the time period during which a specific antibody develops and becomes detectable in the blood.
After two vaccinations, mRNA-1273 elicited robust neutralizing antibody titers. At Day 43, neutralizing activity against SARS-CoV-2 (PRNT80) was seen in all evaluated participants. At the Phase 3 selected dose of 100 µg, the geometric mean titer levels were 4.1-fold above those seen in reference convalescent sera (n=3).
Evaluation of the durability of immune responses is ongoing, and participants will be followed for one year after the second vaccination, with scheduled blood collections throughout that period.
With the Phase 3 dose being finalized at 100 μg, Moderna says it remains on track to be able to deliver approximately 500 million doses per year, and possibly up to 1 billion doses per year, beginning in 2021, due to collaborations with Lonza, Catalent and ROVI.
The Phase 3 study protocol has already been reviewed by the U.S. Food and Drug Administration (FDA) and is expected to include approximately 30,000 participants at the 100 µg dose level in the U.S. The primary endpoint will be the prevention of symptomatic COVID-19 disease with study initiation planned for July 27.
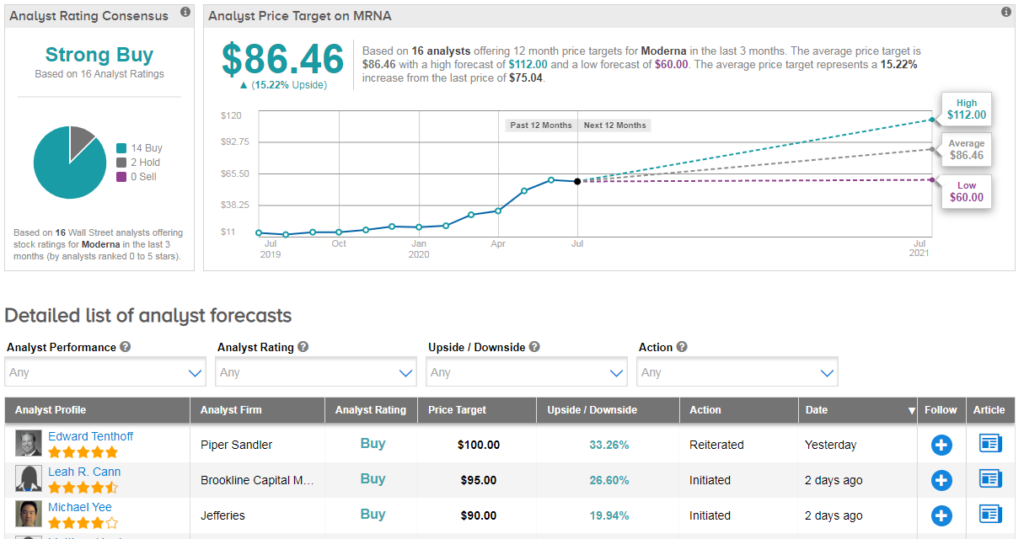
Shares in Moderna have surged 280% so far this year, and Wall Street analysts have a bullish Strong Buy consensus on the stock’s outlook. The $86 average price target suggests an additional 15% upside potential lies ahead. (See MRNA stock analysis).
“With COVID-19 cases rising across large swaths of the US, and a record high of new US cases we believe the stage is set for rapid evaluation of the study’s primary endpoint of symptomatic COVID-19 disease prevention” comments Chardan Capital analyst Geulah Livshits. She has a buy rating on the stock and a $84 price target.
Meanwhile JP Morgan’s Cory Kasimov writes: “The company has spent almost a decade building a world-class platform around mRNA therapeutics, a new class of medicines that, if ultimately successful, could have broad and disruptive potential across the whole biopharma landscape.”
Related News:
IMV Pops 134% In Pre-Market On “Rapid Progress” Of Covid-19 Vaccine Development
Abbott Labs, Edwards Lifesciences Settle Heart Device Patent Disputes
Akebia Initiates Vadadustat Study In Covid-19 Patients









