Shares of Moderna spiked 15% in Monday’s pre-market session as the company disclosed that its vaccine candidate, mRNA-1273, has an efficacy of 94.5% to prevent COVID-19.
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
More specifically, Moderna (MRNA) announced that an independently appointed Data Safety Monitoring Board (DSMB) for the Phase 3 study of mRNA-1273, concluded that the trial has met the statistical criteria pre-specified in the study protocol for efficacy, with a vaccine efficacy of 94.5%.
Following the interim safety and efficacy data, Moderna now plans to file for an Emergency Use Authorization (EUA) with the US Food and Drug Administration (FDA) in the coming weeks and expects the EUA to be based on the final analysis of 151 cases and a median follow-up of more than two months. The company also intends to submit applications for authorizations to other global regulatory agencies.
The study, also known as the COVE study, enrolled more than 30,000 participants in the US and is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), and the Biomedical Advanced Research and Development Authority (BARDA).
“This is a pivotal moment in the development of our COVID-19 vaccine candidate. This positive interim analysis from our Phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said Moderna CEO Stéphane Bancel. “We look forward to the next milestones of submitting for an EUA in the U.S., and regulatory filings in countries around the world, while we continue to collect data on the safety and efficacy of the vaccine in the COVE study.”
This first interim analysis of the trial was based on 95 infections, of which 90 cases of COVID-19 were observed in the placebo group versus 5 cases observed in the mRNA-1273 group, resulting in a point estimate of vaccine efficacy of 94.5%. The 95 COVID-19 cases included 15 older adults (ages 65+) and 20 participants from diverse communities.
By the end of 2020, Moderna expects to have about 20 million doses of mRNA-1273 ready to ship in the US, and remains on track to manufacture 500 million to 1 billion doses globally in 2021.
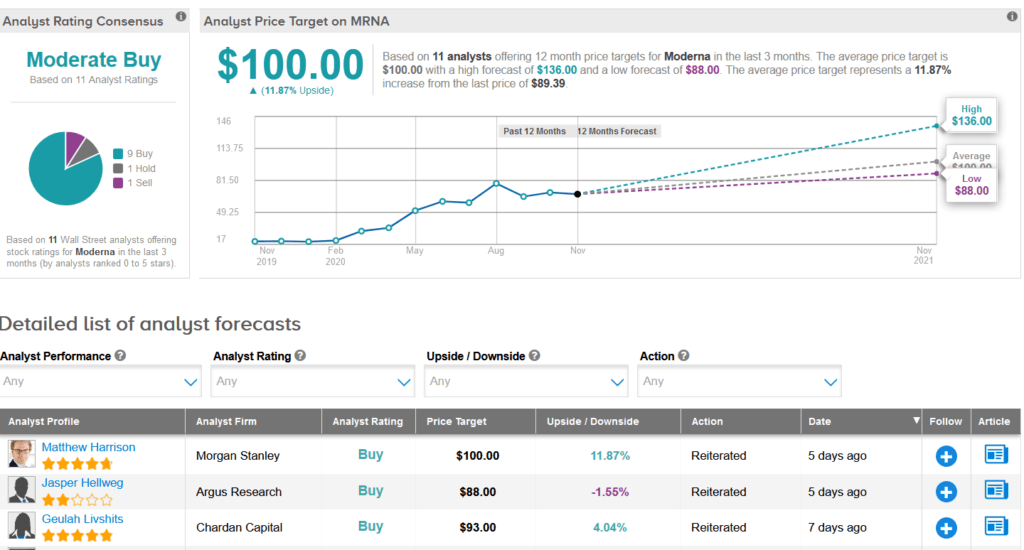
In the race to develop a safe and effective COVID-19 vaccine, shares in Moderna have exploded 357% so far this year. Wall Street analysts still have a Moderate Buy consensus on the stock’s outlook. Looking ahead, the average price target stands at $100, indicating another 12% upside potential lies ahead.
Last week, Argus analyst Jasper Hellweg raised the stock’s price target to $88 from $80 and maintained a Buy rating, citing the company’s continued vaccine progress as it had recently completed the enrollment of 30,000 participants for its Phase 3 study of mRNA-1273.
Hellweg noted that following the accrual and assessment of two months of safety and follow-up data, the company’s next step would be to determine whether to submit an application to the FDA to request EUA. The analyst is also encouraged that Moderna has already inked agreements with multiple governments globally to provide initial doses of mRNA-1273 upon its regulatory approval. (See MRNA stock analysis on TipRanks).
Related News:
J&J Kicks Off Two-Dose Phase 3 Covid-19 Trial In UK; Street Bullish
Pfizer, BioNTech Announce COVID-19 Vaccine is 90% Effective
Ocular Therapeutix Rises 7% On Stellar Results Backed By Dextenza









