Moderna has sealed a deal with the UK government for the supply of another 2 million doses of its COVID-19 vaccine candidate, in addition to the 5 million doses already secured earlier this month. Shares closed 16% higher on Nov. 27.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
According to the agreement with the UK, the additional 2 million doses of Moderna’s (MRNA) mRNA-1273 vaccine candidate against COVID-19 are expected to be supplied in March 2021, assuming they are granted all regulatory approvals. On October 27, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) started the rolling review process for the approval of mRNA-1273.
The candidate is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike (S) protein and is being co-developed with researchers from the National Institute of Allergy and Infectious Disease’s (NIAID) Vaccine Research Center.
“We appreciate the collaboration with the UK government as with many other governments and other key partners around the world,” said Moderna CEO Stéphane Bancel. “For almost a decade, Moderna has invested in creating and developing a novel platform for designing and manufacturing a new class of mRNA-based vaccines. We are proud of the progress on mRNA-1273 we have made to date including the positive interim analysis from our Phase 3 study.”
Last week, Moderna inked a deal to supply European Union countries with up to 160 million doses of its COVID-19 vaccine candidate.
The supply deals come after the biotech company this month disclosed that an independent Data Safety Monitoring Board (DSMB) for the Phase 3 study of its mRNA-1273 concluded that it has an efficacy of 94.5% in preventing COVID-19. In addition, the trial met the statistical criteria pre-specified in the study protocol for efficacy.
Moderna said that it continues to scale up its global manufacturing capacity for the delivery of about 500 million vaccine doses per year and up to 1 billion doses per year, beginning in 2021. Outside of the US, the company is working with its strategic manufacturing partners, Lonza of Switzerland and ROVI of Spain, to supply the countries it has purchase agreements with.
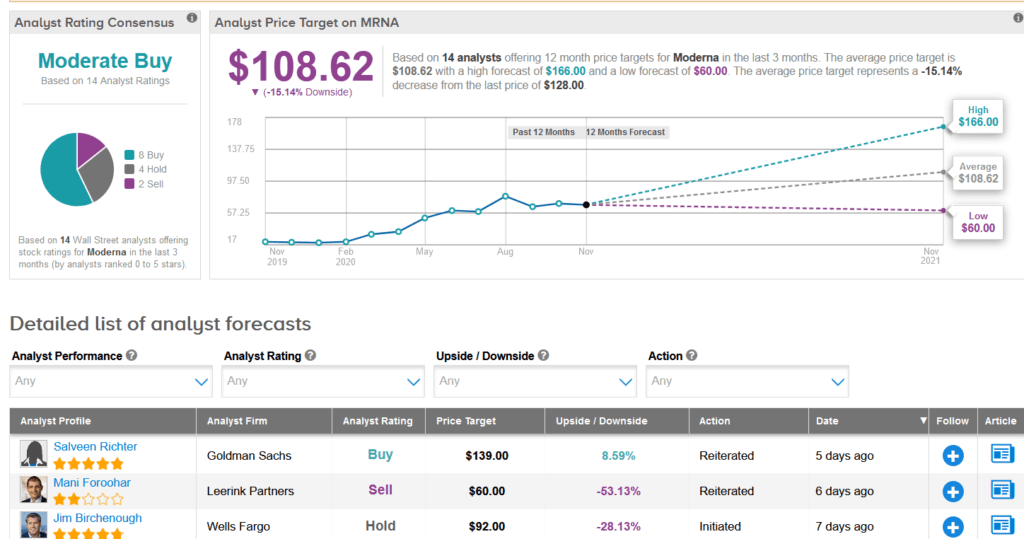
In the race to develop a safe and effective COVID-19 vaccine, shares in Moderna have surged 89% over the past month and are up a whopping 551% so far this year. Meanwhile, Wall Street analysts still have a Moderate Buy consensus on the stock’s outlook. Looking ahead, the average price target stands at $108.62, indicating 15% downside potential lies ahead.
Following the vaccine efficacy data, Goldman Sachs analyst Salveen Richter raised the stock’s price target to $139 from $107 and maintained a Buy rating. Richter expects Moderna’s COVID-19 vaccine to receive US emergency use regulatory approval in December and is confident that the company remains on track to deliver 500 million or up to 1 billion vaccine doses per year, beginning in 2021.
Based on this timeline, the analyst forecasts that Moderna will generate revenue of $305 million and $13.3 billion in Q4 and 2021, respectively, from the supply of mRNA-1273. (See MRNA stock analysis on TipRanks).
Related News:
Fulgent Genetics Lifts 2020 Sales Outlook; BTIG Flips To Hold
Moderna Inks EU Supply Deal For 160M Covid-19 Vaccine Doses
Regeneron’s Covid-19 Antibody Cocktail Wins FDA Emergency Use Nod