Moderna has inked a deal to supply European Union countries with up to 160 million doses of its COVID-19 vaccine candidate.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
The supply deal comes after Moderna (MRNA) last week disclosed that an independent Data Safety Monitoring Board (DSMB) for the Phase 3 study of its mRNA-1273 vaccine candidate concluded that it has an efficacy of 94.5% in preventing COVID-19. In addition, the trial met the statistical criteria pre-specified in the study protocol for efficacy.
“According to the results of clinical trials, this vaccine could be highly effective against COVID-19,” European Commission President Ursula von der Leyen said in a statement. “Once the vaccine is indeed proven as safe and effective, every Member State will receive it at the same time, on a pro-rata basis, at the same conditions.”
Von der Leyen added that the Moderna deal marks the sixth contract the EU has struck with a pharmaceutical company for the supply of COVID-19 vaccines. “We are working on yet another one,” she stated, without providing any details.
Following the recent interim safety and efficacy data, Moderna plans to file for an Emergency Use Authorization (EUA) with the US Food and Drug Administration (FDA) in the coming weeks and expects the EUA to be based on the final analysis of 151 cases and a median follow-up of more than two months. The company also intends to submit applications for authorizations to other global regulatory agencies.
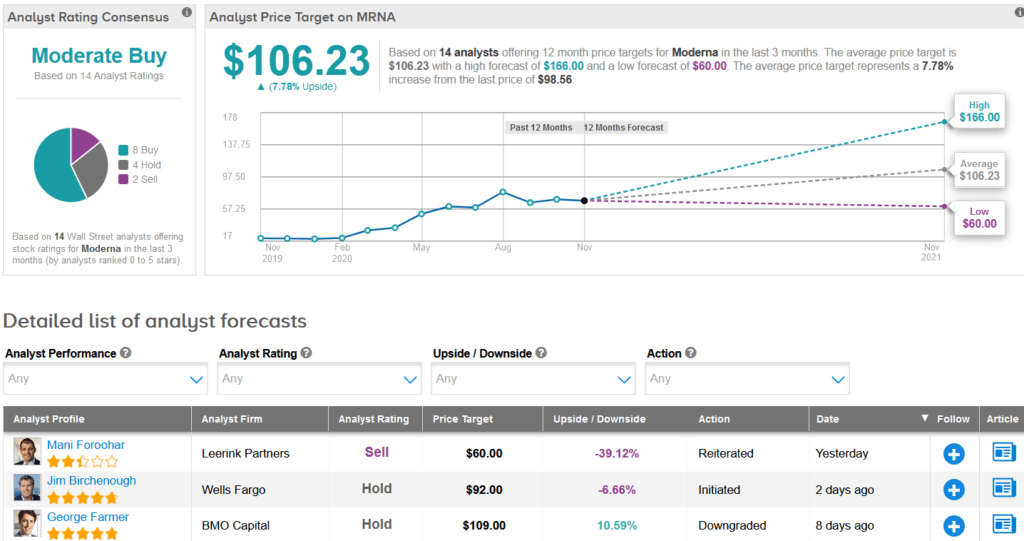
In the race to develop a safe and effective COVID-19 vaccine, shares in Moderna have exploded 404% so far this year. Wall Street analysts still have a Moderate Buy consensus on the stock’s outlook. Looking ahead, the average price target stands at $106.23, indicating another 8% upside potential lies ahead.
Meanwhile, Wells Fargo analyst Jim Birchenough this week, initiated coverage of the stock with a Hold rating and a $92 price target.
Birchenough noted that Moderna’s recent COVID-19 vaccine results, which suggest a 94.5% reduction in infection risk are “compelling and should support a strong public health benefit.” However, the analyst remains cautious amid concern about the vaccine’s potency and durability of protection over time. He also cited potential supply chain, distribution, and competitive pressures as risk factors. (See MRNA stock analysis).
Related News:
Regeneron’s Covid-19 Antibody Cocktail Wins FDA Emergency Use Nod
FDA To Review Pfizer-BioNTech Covid-19 Vaccine On Dec. 10
Eiger Pops 12% On FDA Approval For First Hutchinson-Gilford Progeria Treatment