Moderna Inc. (MRNA) has entered into an agreement with Spain’s Laboratorios Farmacéuticos Rovi, S.A. for large-scale manufacturing and supply of its mRNA-based experimental COVID-19 vaccine candidate outside of the U.S.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Following the news shares rose 5.5% to $64.97 at the close on Thursday. As part of the agreement, Rovi will procure a new product line to provide vial filling and packaging, equipment for compounding, automatic visual inspection and labeling for the production of hundreds of millions of doses of the vaccine candidate to supply markets outside of the U.S. starting in early 2021, the two companies said in a statement.
Rovi will also hire additional staff required to boost manufacturing operations and production.
“We are pleased to partner with Rovi to potentially supply hundreds of millions of doses of finished mRNA-1273, once approved, and help address the need for a vaccine against COVID-19 around the world,” said Juan Andres, Moderna’s CTO and Quality Officer. “Rovi’s experience as a global manufacturer of drug product and expertise in fill-finish will be an important partnership for us to establish dedicated supply chains that can meet the needs of different countries and regions.”
Moderna, which in recent weeks signed agreements with Lonza and Catalent (CTLT) to scale up manufacturing, said this week that it remains on track to be able to deliver about 500 million doses per year, and possibly up to 1 billion doses per year, beginning in 2021.
On Wednesday, the biotech company announced that it has completed enrollment for the Phase 2 study of its mRNA-1273 vaccine candidate. The clinical study will assess the safety, reactogenicity, and immunogenicity of 2 dose levels of mRNA-1273 in around 600 adults 18 years of age or older.
According to the trial record, the estimated completion date will be August 2021 with the estimated primary completion date expected for March 2021.
The company said that the cohorts of older adults and elderly adults in the NIH-led Phase 1 study have completed enrollment. Results are expected to be published once available. Moderna has also now finalized the Phase 3 study protocol based on feedback from the U.S. Food and Drug Administration (FDA).
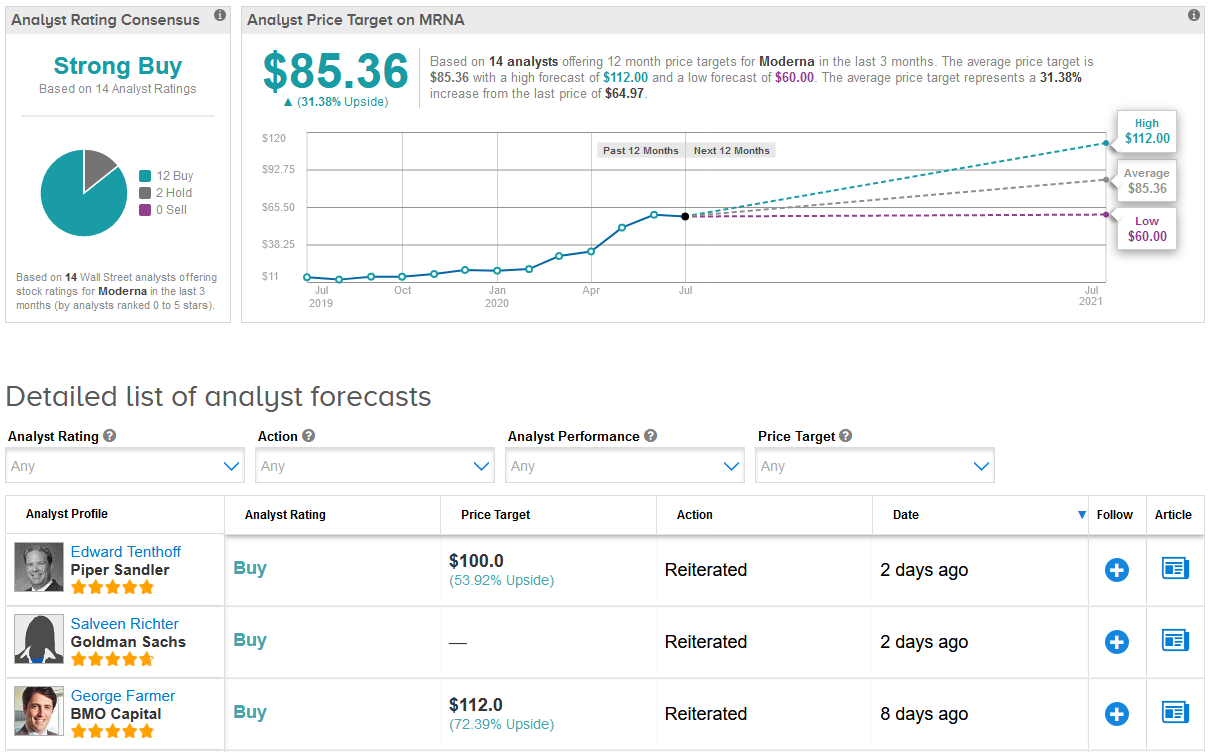
Shares in Moderna have more than tripled this year, as investors piled into the stock betting on the potential of its virus vaccine. Indeed, Wall Street analysts have a bullish Strong Buy consensus on the stock’s outlook. What’s more, the $85.36 average price target suggests an additional 31% upside potential lies ahead. (See MRNA stock analysis on TipRanks).
“With COVID-19 cases rising across large swaths of the US, and a record high of new US cases we believe the stage is set for rapid evaluation of the study’s primary endpoint of symptomatic COVID-19 disease prevention” comments Chardan Capital analyst Geulah Livshits. She has a buy rating on the stock and a $84 price target.
Meanwhile JP Morgan’s Cory Kasimov writes: “The company has spent almost a decade building a world-class platform around mRNA therapeutics, a new class of medicines that, if ultimately successful, could have broad and disruptive potential across the whole biopharma landscape.”
Related News:
Novavax Spikes 42% Pre-Market On $1.6B U.S. Funding For Covid-19 Candidate
Gilead’s Covid-19 Remdesivir Therapy Gets Conditional European Nod
Can Novavax Win FDA Approval for COVID-19 Vaccine Before Year End? Analyst Weighs In