Moderna confirmed that it is engaged in discussions with the Ministry of Health, Labour and Welfare of Japan (MHLW) to potentially purchase 40 million or more doses of its mRNA-1273 vaccine candidate against COVID-19.
Under the terms of the arrangement, the vaccine would be supplied by Moderna (MRNA) and distributed in Japan by Takeda Pharmaceutical Co., Ltd. beginning in the first half of 2021, if the vaccine receives regulatory approval and proves to be safe and efficient.
Kato Katsunobu, Minister of MHLW, announced the discussions in a meeting with media in Japan on Friday. The talks come as Japan seeks to provide vaccines to the public as soon as possible. Meanwhile, Japan has already signed agreements with other drugmakers such as Pfizer and AstraZeneca.
Last week, Moderna announced that it concluded advanced exploratory talks with the European Commission to supply 80 million doses of mRNA-1273. Earlier this month the US government committed $1.5 billion to receive 100 million doses of its COVID-19 vaccine candidate.
mRNA-1273 is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike (S) protein, and is being co-developed by Moderna and investigators from the National Institute of Allergy and Infectious Disease’s (NIAID) Vaccine Research Center.
On Thursday, Moderna disclosed that its mRNA-1273 vaccine in the Phase 1 study demonstrated robust immune responses in older adults that were comparable to those in younger adults.
The company had also announced that the Phase 3 COVE study of mRNA-1273 began on July 27; enrollment is on track to complete in September. As of Aug. 25, 15,239 participants have been enrolled.
Moderna reiterated that it remains on track to deliver about 500 million doses per year, and up to 1 billion doses per year, beginning in 2021. Initial funding of $1.3 billion for Moderna to begin producing mRNA-1273 was secured from investors in the company’s most recent public equity offering in May 2020.
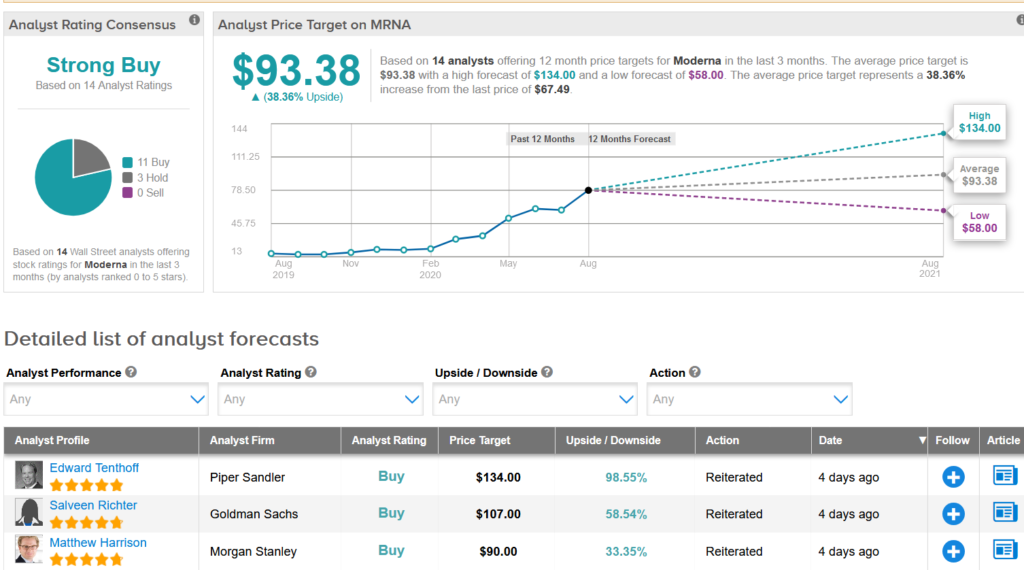
Shares in Moderna have dropped 9% over the past month but have exploded 245% so far this year. Wall Street analysts still have a Strong Buy consensus on the stock’s outlook. Looking ahead, the $93.38 average price target suggests an additional 38% upside potential lies ahead.
Commenting on the latest Phase 1 data, JPMorgan analyst Cory Kasimov said that although the vaccine candidate appears to be showing a “promising profile”, he still wants to see “the Phase 3 results with mRNA-1273, which are anticipated sometime this fall, to see how the data evolves.”
Kasimov maintains a Hold rating on the stock as he is somehow sceptical of Moderna’s “ability to generate long-term meaningful revenues that justify prevailing market values.” (See MRNA stock analysis on TipRanks)
Related News:
Moderna Pops 6% On Covid-19 Vaccine Response In Older Adults; Analyst Says Buy Now
AstraZeneca Rises On Report Trump Could Fast-Track Covid-19 Vaccine Candidate
Abbott Expanding Its Covid-19 Test To Asymptomatic People- Report
















