Johnson & Johnson expects to have all the data needed to seek U.S. authorization for its experimental COVID-19 vaccine by February or earlier, the US drugmaker’s chief scientist Paul Stoffels told Reuters.
J&J’s (JNJ) Stoffels said that the drugmaker is recruiting over 1,000 people per day for the late-stage trial of its COVID-19 vaccine candidate.
“By the end of the year or around the end of the year, we should have 60,000 people in the study,” Stoffels commented in an interview with Reuters. “And efficacy endpoint should be there in the first few weeks or months, January or February, of the new year.”
The Phase 3 trial of the single-dose vaccine, Ad26.COV2-S, was initiated in late September. Ad26.COV2-S is currently undergoing clinical trials after they were briefly halted last month following a serious illness in a study participant. On Oct. 23, J&J announced the resumption of the Phase 3 US trial of its COVID-19 vaccine candidate, after “no evidence” was found that the vaccine caused the illness.
“In a pandemic a single shot is definitely important globally,” Stoffels said. “(A two-shot vaccine) is a very significant operational challenge. More so in healthcare systems which are less well organized.”
Single-shot vaccines will likely be beneficial in particular remote areas, Stoffels said.
J&J will need to provide safety data to the US Food and Drug Administration (FDA) for at least one-half of trial participants for the two-month period after they receive the vaccine. “So that will bring us around the year end or early next year for having all the data,” he added.
Meanwhile, rival drugmakers Moderna and Pfizer, already released interim data from late-stage clinical trials of their vaccine candidates this month, which showed that they were more than 90% effective in preventing COVID-19.
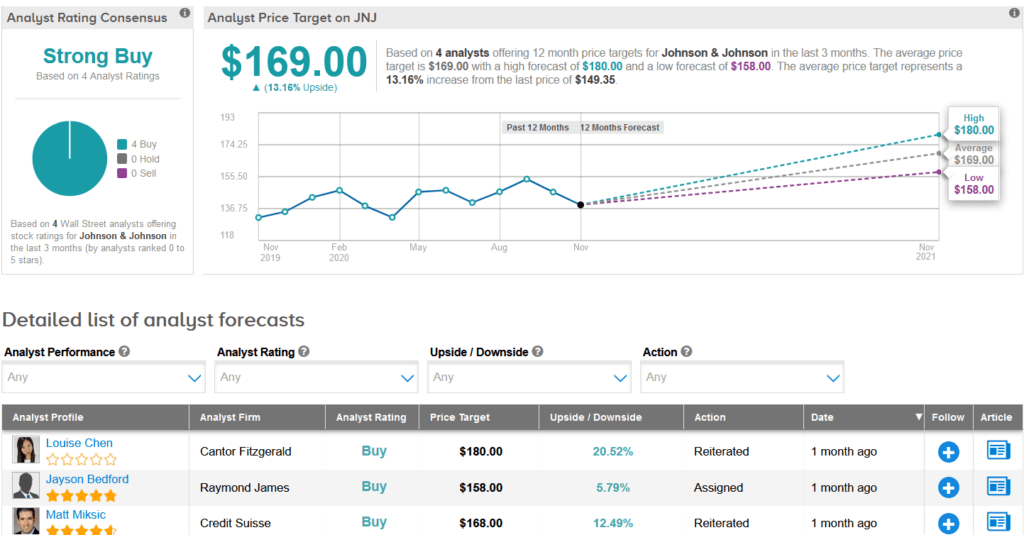
Shares of JNJ are up 2.4% year-to-date, and the stock scores a bullish Strong Buy Street consensus. That’s with 4 back-to-back Buy ratings over the last three months. Meanwhile, the average analyst price target of $169 indicates 13% upside potential lies ahead.
Last month, Cantor Fitzgerald analyst Louise Chen raised the stock’s price target to $180 (21% upside potential) from $168 and maintained a Buy rating, saying that J&J’s recent Q3 EPS and sales beat reflect solid performance and positive trends across its diversified business model. (See JNJ stock analysis on TipRanks)
Related News:
Pfizer Kicks Off Covid-19 Vaccine Pilot Delivery Program In US – Report
BioNTech, Fosun Pharma Get Green Light To Start Covid-19 Vaccine Trial In China
Moderna Says Covid-19 Vaccine Candidate 94.5% Effective; Shares Pop 15%
















