Johnson & Johnson (JNJ) said on Wednesday that it will start human trials of its COVID-19 vaccine as early as in the second half of July. The Phase 1/2 human clinical trial of its investigational SARS-CoV-2 vaccine, also known as Ad26.COV2-S, was scheduled to begin in September.
The news sent shares up 2.3% to $149.40 in midday trading. J&J said it already has supply agreements to produce more than 1 billion doses of the vaccine candidate globally through the course of 2021, should it prove to be safe and successful. There is currently no approved vaccine for COVID-19.
“Based on the strength of the preclinical data we have seen so far and interactions with the regulatory authorities, we have been able to further accelerate the clinical development of our investigational SARS-CoV-2 vaccine,” said Paul Stoffels, Chief Scientific Officer at J&J. “Simultaneously, we are continuing our efforts to build important global partnerships and invest in our vaccine production technology and manufacturing capabilities.”
The randomized, double-blind, placebo-controlled Phase 1/2 study will test and examine the safety, response to vaccination, and immunogenicity of the investigational Ad26.COV2-S vaccine, in 1,045 healthy adults aged 18 to 55 years, as well as adults aged 65 years and older. The study will be conducted in the U.S. and Belgium.
Moreover, J&J is in talks with the National Institutes of Allergy and Infectious Diseases to start the Phase 3 clinical trial of its Ad26.COV2-S vaccine candidate, ahead of its original schedule, subject to the outcome of the Phase 1 studies and regulatory approval.
The development and production of J&J’s SARS-CoV-2 vaccine candidate is supported by a collaboration between Janssen Pharmaceutical Cos and the U.S. Biomedical Advanced Research and Development Authority (BARDA).
Shares in J&J have been on a gaining streak since plunging to a multi-year low in March and are now trading some 3% higher than at the start of the year.
UBS analyst Kevin Caliendo recently cut the stock to Hold from Buy with a $160 price target, as he believes that drug sales over the next few years will be lower than the market anticipates.
“The stock is trading near a historical high relative multiple on 2021,” Caliendo wrote in a note to investors. “We believe upside from here depends more on a view of market valuation than [J&J]’s fundamentals.”
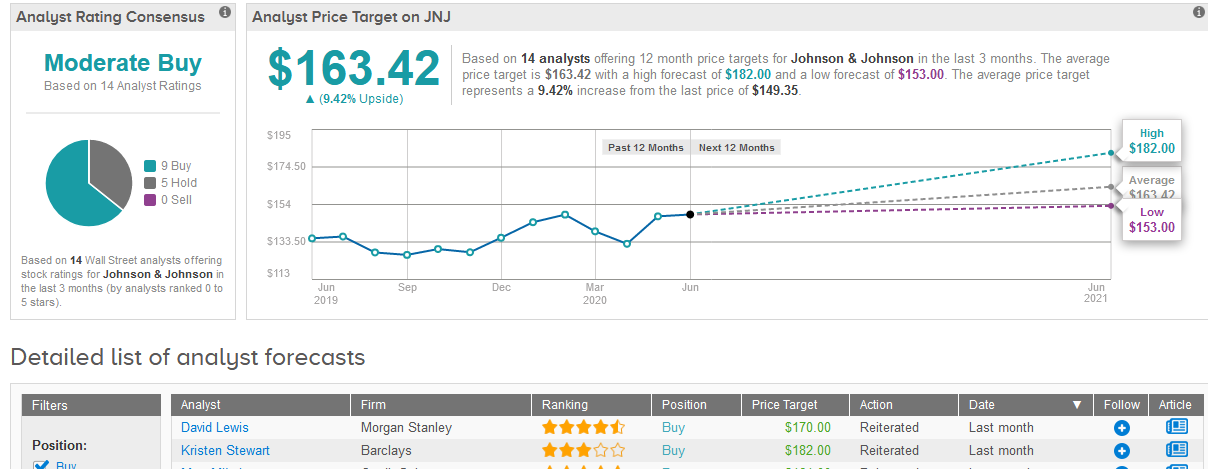
Overall the stock shows a cautiously optimistic Moderate Buy analyst consensus with 9 Buy ratings versus 5 Hold ratings. That’s with a $163.42 average analyst price target indicating upside potential of 9.4% in the coming 12 months. (See JNJ stock analysis on TipRanks).
Related News:
5 Promising Covid-19 Vaccines Picked For Trump’s Operation Warp Speed
What Would a Merger Mean for Gilead? Top Analyst Weighs In
Soleno Plunging 48% In Pre-Market On Obesity Study Failure