Gilead Sciences Inc. (GILD) announced on Friday that the European Commission has granted its experimental drug candidate remdesivir conditional marketing authorization for the treatment of SARS-CoV-2 infection, the virus that causes COVID-19.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Remdesivir is the first approved potential therapy option for the treatment of COVID-19 in the European Union. The conditional authorization was granted following a rolling review of supporting data that began in April 2020, Gilead said.
The authorization is for the use of remdesivir in the treatment of COVID-19 in adults and adolescents aged 12 years and older, who also suffer from pneumonia and need supplemental oxygen.
“We appreciate the European Medicines Agency’s rapid review of remdesivir in recognition of the unprecedented nature of this pandemic,” said Gilead’s Chief Medical Officer Merdad Parsey. “This conditional marketing authorization is an important step forward as we work together to address the treatment needs of patients across Europe.”
The conditional marketing authorization in Europe is initially valid for one year but can be extended or converted into an unconditional marketing authorization after the submission and assessment of additional data.
Ongoing clinical trials continue to evaluate the safety and efficacy of remdesivir, including studies of the potential treatment in combination with anti-inflammatory medicines and in special populations including pediatric patients, Gilead added. Research is also being conducted on new, investigational formulations of remdesivir that may enable studies of the antiviral drug in earlier stages of disease.
Remdesivir is a viral RNA polymerase inhibitor which means that it interferes with the production of viral genetic material, preventing the virus from multiplying. Gilead’s investigational antiviral therapy has received Emergency Use Authorization (EUA) by the U.S Food and Drug Administration to treat COVID-19. It has also been approved as a treatment for patients with severe COVID-19 in Japan, Taiwan, India, Singapore, the United Arab Emirates.
The European approval comes after Gilead last week priced remdesivir at $2,340 per patient for a 5-day treatment in the U.S. and other developed countries.
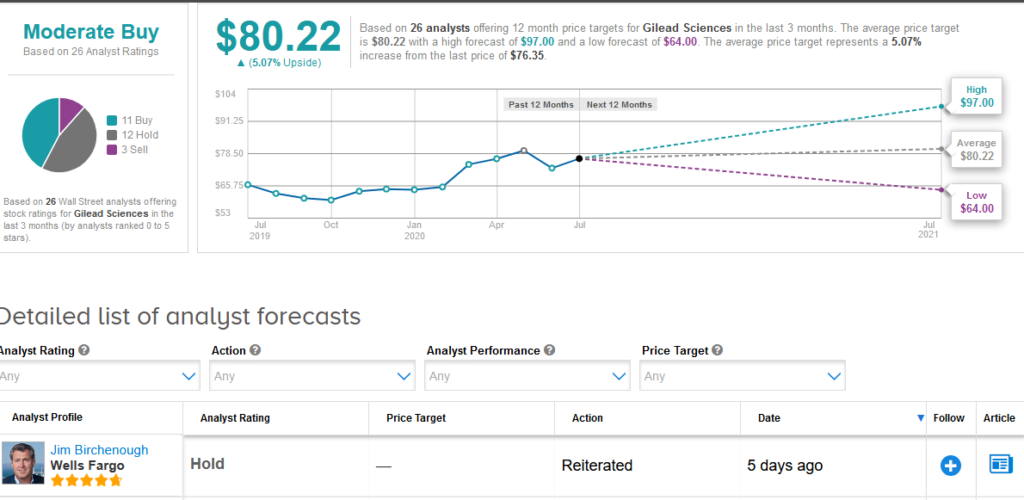
Five-star analyst Jim Birchenough at Wells Fargo reiterated a Hold rating on the stock saying that although pricing is below the high end of recommendations and represents “a responsible public health decision”, he remains skeptical on the longer-term commercial opportunity in view of emerging competition and potential vaccine progress.
The rest of the Street is cautiously optimistic on the stock. The Moderate Buy analyst consensus is based on 11 Buy ratings versus 12 Hold ratings and 3 Sell ratings.
Shares in Gilead rose less than 1% to $76.35 at the close of trading on July 2 taking the year-to-date advance to about 17%. The $80.22 average price target implies 5.1% upside potential in the shares in the coming 12 months. (See Gilead stock analysis on TipRanks).
Related News:
Sanofi, Regeneron’s Covid-19 Kevzara Trial Misses Endpoints; Shares Fall
Gilead Sets Pricing for Covid-19 Treatment Remdesivir at $2,340 Per Patient
Moderna Sinks 5% On Covid-19 Vaccine Delay Reports









