Gilead Sciences (GILD) has announced that the U.S. Food and Drug Administration (FDA) has approved the antiviral drug Veklury (remdesivir) for the treatment of patients with COVID-19 requiring hospitalization. Shares are surging 7% in Friday’s pre-market trading.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
An antiviral drug, Veklury works to stop replication of SARS-CoV-2, the virus that causes COVID-19. Previously authorized by the FDA for emergency use to treat COVID-19, Veklury is now the first and only approved COVID-19 treatment in the US.
The FDA approved Veklury for patients 12 years+ and over 40kg, and instructs that the drug should only be administered in a hospital or in a healthcare setting capable of providing acute care.
The approval is based on three randomized controlled trials including the recent NIAID double blind, placebo-controlled Phase 3 ACTT-1 trial, which showed that treatment with Veklury resulted in clinically meaningful improvements compared with placebo.
For instance, Veklury significantly improved time to recovery vs placebo – by five days in the overall study population and seven days in patients who required oxygen support. As a secondary endpoint, Veklury also reduced disease progression in patients needing oxygen, resulting in a significantly lower incidence of new mechanical ventilation or ECMO (13% vs. 23%). There was also a trend toward reduced mortality with Veklury vs placebo at Day 29 (11.4% vs. 15.2%).
“Since the beginning of the COVID-19 pandemic, Gilead has worked relentlessly to help find solutions to this global health crisis. It is incredible to be in the position today, less than one year since the earliest case reports of the disease now known as COVID-19, of having an FDA-approved treatment in the U.S. that is available for all appropriate patients in need,” said Daniel O’Day, Gilead CEO.
In parallel, the FDA also issued a new Emergency Use Authorization (EUA) for Veklury to treat hospitalized pediatric patients under 12 years of age weighing at least 3.5 kg.
Gilead says that the drug is now widely available in hospitals across the country, following early investments to rapidly expand manufacturing capacity to increase supply. It anticipates meeting global demand for Veklury in October, even in the event of potential future surges of COVID-19.
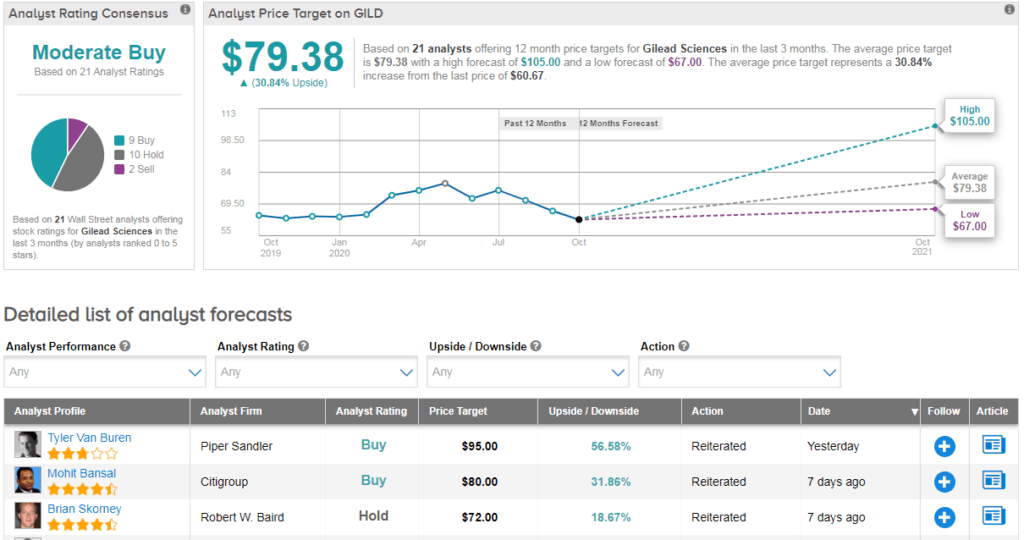
Shares in Gilead have declined about 7% year-to-date with the $79 average analyst price target indicating 31% upside potential lies ahead in the coming 12 months.
Piper Sandler analyst Tyler Van Buren reiterated his buy rating on GILD and $95 price target following the news. The analyst writes that he would be “curious to learn more about post-marketing requirements that have been agreed upon”, but nonetheless believes the drug could deliver $1B+ in sales for Gilead during 2H20 if the current case rate continues. He adds that the decision reflects the FDA’s confidence in remdesivir’s safety- especially given the emergency approval for children.
The rest of the Street is cautiously optimistic on the stock. The Moderate Buy analyst consensus is based on 9 Buys versus 10 Holds and 2 Sells. (See Gilead stock analysis on TipRanks).
Related News:
Xeris Wins FDA Fast Track Path For Epilepsy Treatment; Shares Spike 9%
AbbVie Submits US, EU Rinvoq Applications For Atopic Dermatitis
BD’s Covid-19-Flu Combo Test Wins European Clearance









