Gilead Sciences Inc.’s (GILD) signed non-exclusive licensing agreements with five generic pharmaceutical manufacturers that will allow them to produce and sell its experimental remdesivir coronavirus drug in 127 countries, including India and Pakistan.
Under the terms of the licensing agreements, the generic drugmakers – Cipla Ltd., Ferozsons Laboratories, Hetero Labs Ltd., Jubilant Lifesciences and Mylan – based in India and Pakistan will be entitled to tap the technological know-how of Gilead’s manufacturing process to enable them to scale up production more quickly.
“We will be monitoring the clinical trials and regulatory approvals very closely and would be ready to launch the drug shortly after the required regulatory approvals,” Shyam S. Bhartia, Co-Chairman and Managing Director at India’s Jubilant Life Sciences. “We also plan to produce the drug’s Active Pharmaceutical Ingredient (“API”) in-house helping its cost effectiveness and consistent availability.”
The countries for distribution of the experimental remdesivir coronavirus drug consist of nearly all low-income and middle income countries, as well as several upper-middle- and high-income countries, including South Africa and North Korea, that face significant obstacles to healthcare access.
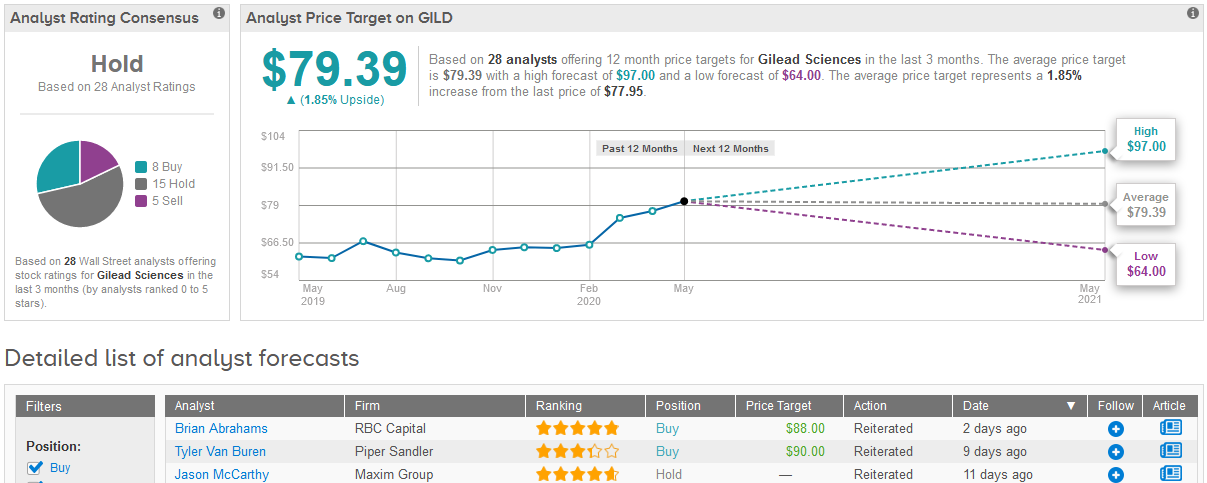
Shares in Gilead fell 3.5% to $77.95 on Tuesday trimming this year’s rally to about 20%.
Five-star analyst Cory Kasimov at J.P. Morgan maintained his Hold rating on the stock with a $85 price target following a call with the company’s management.
“Although we’re impressed by the company’s intensive work with remdesivir, we believe that GILD received ample market credit for it and remdesivir is unlikely to result in tangible long-term cash flows,” Kasimov wrote in a note to investors. “A short-term $5 billion boost sales has marginal impact on our overall valuation and other opportunities for growth will take time to play out.”
TipRanks data shows that out of the 28 analysts covering Gilead in the past three months, 15 are now sidelined with a Hold rating on the stock, 8 say Buy and 5 say Sell, adding up to a Hold consensus rating. The $79.39 average price target suggests analysts see very limited upside potential in the shares in the coming 12 months. (See Gilead stock analysis on TipRanks)
Last week Gilead said that it was also in talks with drugmakers in Europe for supply pacts for its remdesivir COVID-19 drug candidate. The antiviral drug was this month granted emergency use authorization by the U.S. Food and Drug Administration to treat COVID-19 patients.
According to the licensing agreements, the drugmakers will set their own prices for the generic product they produce, Gilead said. The licenses are royalty-free until the World Health Organization declares the end of the Public Health Emergency of International Concern regarding COVID-19, or until a pharmaceutical product other than remdesivir or a vaccine is approved to treat or prevent the virus, whichever is earlier.
Related News:
Moderna’s Covid-19 Vaccine Candidate Gets FDA Fast Track Status
Novavax Spikes 31% on $384 Million Cash Injection for Vaccine Production
CymaBay Doubles After-Hours On Positive NASH Trial Update