The European Commission announced Thursday that it has concluded exploratory talks with US pharmaceutical company Johnson & Johnson to secure the initial purchase of 200 million doses of its potential vaccine against COVID-19.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The concluded talks represent part of a proposed contract with Johnson & Johnson (JNJ), which would allow all European member states to buy the vaccine, as well as providing them with the option to donate to lower and middle income countries or re-direct to European Economic Area countries, the commission said in a statement.
It was agreed that once a vaccine has proven to be safe and effective against COVID-19, the commission would have a contractual framework in place for the initial purchase of 200 million doses on behalf of all EU member states, with an option to purchase an additional 200 million vaccine doses. Financial terms weren’t disclosed. The commission said that it is also pursuing “intensive” talks with other vaccine manufacturers.
“We are deeply committed to providing global access to our SARS-CoV-2 vaccine candidate. That’s why, as we proceed with development of our vaccine, we are simultaneously working with partners around the world including the European Commission and member states to help us reach that goal,” said Paul Stoffels, Johnson & Johnson’s Vice Chairman of the Executive Committee.
Last week, Johnson & Johnson entered into an agreement with the US government for large scale domestic manufacturing and supply of 100 million doses of its SARS-CoV-2 investigational vaccine, Ad26.COV2.S, for more than $1 billion.
At the end of last month, the US pharmaceutical company announced the start of first-in-human safety trials of its COVID-19 vaccine candidate after a single dose in pre-clinical studies showed “robust protection”.
Johnson & Johnon has said that as the company progresses the clinical development of its SARS-CoV-2 vaccine candidate, it continues to boost manufacturing capacity and is in active discussions with global strategic partners to support worldwide access. This is part of the company’s target to supply more than 1 billion doses globally through the course of 2021, provided the vaccine is safe and effective.
Shares have recovered since plunging to a multi-year low in March and are now trading 1.7% higher than at the start of year. (See JNJ stock analysis on TipRanks).
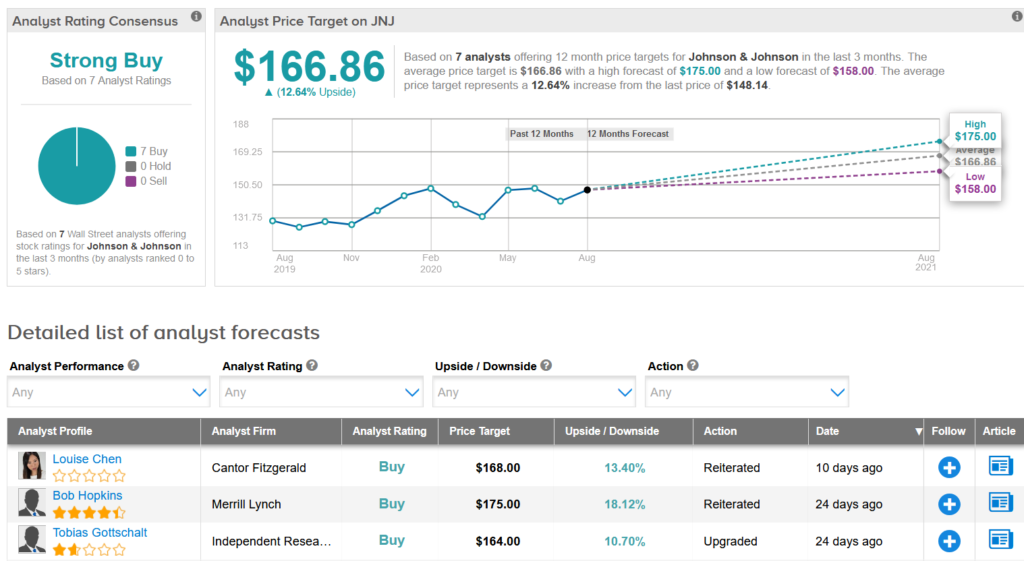
Late last month, Merrill Lynch analyst Bob Hopkins reiterated a Buy rating on the stock with a $175 price target (18% upside potential), saying that he continues to like the risk reward in JNJ.
“While visibility remains low with litigation, COVID’s impact on the economy could well bring names like JNJ back into favor and goodwill from a successful vaccine could limit drug pricing risk,” Hopkins wrote in a note to investors. “The stock trades at a discount to the S&P 500 which seems a relative mispricing given JNJ’s strong relative outlook in this challenging time and JNJ should be able to deliver strong results through a pressured environment.”
Overall, the rest of the Street shares Hopkins’ bullish outlook on the stock. The Strong Buy analyst consensus boasts 7 unanimous Buy ratings. That’s with a $166.86 average price target indicating upside potential of 13% in the coming 12 months.
Related News:
Quidel’s Lyra Covid-19 Test Gets Nod For Sale In Canada; Shares Rise 6%
Argentina, Mexico To Produce AstraZeneca’s Covid-19 Vaccine Candidate
Pfizer Inks Deal To Manufacture Gilead’s Covid-19 Remdesivir Treatment









