Emergent BioSolutions Inc. (EBS) said on Thursday that it signed an agreement to manufacture AstraZeneca Plc’s (AZN) potential COVID-19 vaccine in the U.S. in a deal valued at $87 million.
Under the terms of the manufacturing agreement, Emergent will provide development services, technology transfer, analytical testing, drug substance process and performance qualification and will reserve certain large-scale manufacturing capacity through 2020.
The agreement comes after AstraZeneca said last week that it signed supply chain deals for the capacity to produce 2 billion doses of its potential coronavirus vaccine, also known as AZD1222, which it is developing with Oxford University.
AZD1222, is among a list of several candidates supported by Operation Warp Speed (OWS), the U.S. government program to accelerate the development, manufacturing, and distribution of COVID-19 vaccines available for Americans by Jan. 2021.
As part of OWS, Emergent will provide development and manufacturing services and capacity to companies of leading COVID-19 vaccine candidates selected by the U.S. government, such as AstraZeneca. Separately, Emergent is also developing two investigational plasma-based coronavirus treatments – COVID-Human Immune Globulin (COVID-HIG) and COVID-Equine Immune Globulin (COVID-EIG).
“By partnering with leading innovators like AstraZeneca, Emergent is playing a critical role in increasing the world’s chances of having a safe and effective COVID-19 vaccine,” said Robert G. Kramer, Emergent’s President and CEO. “With this agreement, we bring to our facilities two of the five leading candidates being developed with U.S. government funding.”
Development services will be conducted out of Emergent’s Gaithersburg product development facility. Large-scale manufacturing of drug substance will be done at the Baltimore Bayview facility, an HHS-designated Center for Innovation in Advanced Development and Manufacturing (CIADM) designed for rapid manufacturing of large quantities of vaccines and treatments during public health emergencies.
The CIADM has the capacity to produce tens to hundreds of millions of doses of vaccine on an annual basis, based upon the platform technology being used, Emergent said.
AstraZeneca shares fell 3.2% to $51.93 in early afternoon trading. Shares in Emergent declined 2.2% to $69.68 trimming this year’s advance to 28%.
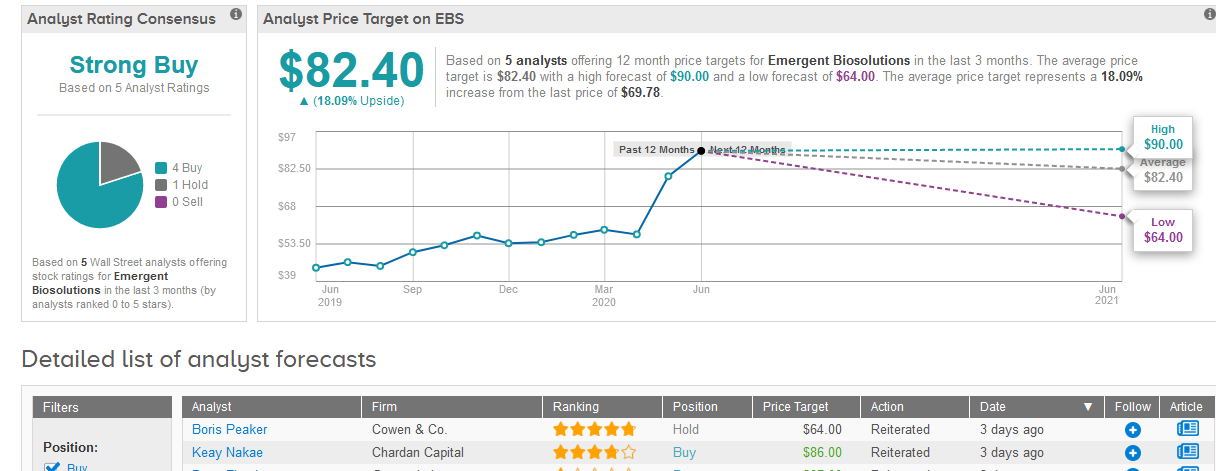
Four-star analyst Keay Nakae at Chardan Capital this week reiterated a Buy rating on the stock with a $86 price target, saying that Emergent has established itself as a preferred supplier of biodefense vaccines and other products to the U.S. government under multiyear sales contracts.
“We believe that the company’s ability to land several new manufacturing contracts since the beginning of this year is just another example of one of Emergent’s key strengths, its longstanding relationships with the relevant government agencies,” Nakae said in a note to investors.
Overall, the stock boasts 4 Buy ratings versus 1 Hold rating adding up to a Strong Buy consensus. Meanwhile, the $82.40 average analyst price target implies another 18% upside potential for the shares in the next 12 months. (See EBS stock analysis on TipRanks).
Related News:
Oxford Biomedica Clinches Manufacturing Deal For AstraZeneca’s Covid-19 Vaccine
5 Promising Covid-19 Vaccines Picked For Trump’s Operation Warp Speed
What Would a Merger Mean for Gilead? Top Analyst Weighs In