Eli Lilly & Co. has struck a $375 million agreement with the US government to supply 300,000 vials of its bamlanivimab (LY-CoV555), an investigational neutralizing antibody, for the treatment of COVID-19. Shares are advancing 1.7% in Thursday’s pre-market trading.
According to the agreement, the US government will receive the LY-CoV555 vials once it has been granted Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA). Earlier this month, Lilly (LLY) submitted an FDA request for EUA approval of the investigational treatment used for mild to moderate COVID-19 in high-risk patients.
The initial agreement is for supply over the 2 months following an EUA. After that, the US government has the option to purchase up to an additional 650,000 vials through June 30, 2021, under the same terms and subject to product availability and the medical need in the US.
Stay Ahead of the Market:
- Discover outperforming stocks and invest smarter with Top Smart Score Stocks
- Filter, analyze, and streamline your search for investment opportunities using Tipranks' Stock Screener
LY-CoV555 is a potent, neutralizing IgG1 monoclonal antibody (mAb) directed against the spike protein of SARS-CoV-2. It is designed to block viral attachment and entry into human cells, thus neutralizing the virus, potentially preventing and treating COVID-19.
“Supply agreements with governments – such as this one with the US government to meet Operation Warp Speed goals – are fundamental to enable the most widespread and equitable access to our potential therapy,” said Lilly CEO David A. Ricks. “The US is experiencing a surge in COVID-19 cases and associated hospitalizations, and we believe bamlanivimab could be an important therapeutic option that can bring value to the overall healthcare system, as it has shown a potential benefit in clinical outcomes with a reduction in viral load and rates of symptoms and hospitalizations.”
Lilly targets manufacturing of up to 1 million doses of bamlanivimab 700 mg by the end of 2020 – with 100,000 doses ready to ship within days of EUA authorization – for use around the world. The supply of Lilly’s antibody therapy is expected to increase substantially beginning in Q1 2021, as additional manufacturing resources come online throughout the year.
The drugmaker said that it has a robust, global supply chain in place to produce LY-CoV555 at 5 manufacturing sites worldwide. To ensure rapid access of the treatment to patients around the world, Lilly has invested in large-scale manufacturing of LY-CoV555, even before data demonstrated its potential to become a meaningful therapeutic option for COVID-19, the company added.
However, the antibody treatment suffered a setback earlier this week after data found that it was “unlikely” to help COVID-10 hospitalized patients recover from the advanced stage of the disease. As a result, the US-based Phase 3 clinical trial for hospitalized patients was stopped, while all other ongoing trials for its coronavirus antibody treatments will be continued.
Shares of LLY have dropped more than 7% over the past 5 days and are trading close to their start of the year level. (See Eli Lilly’s stock analysis on TipRanks).
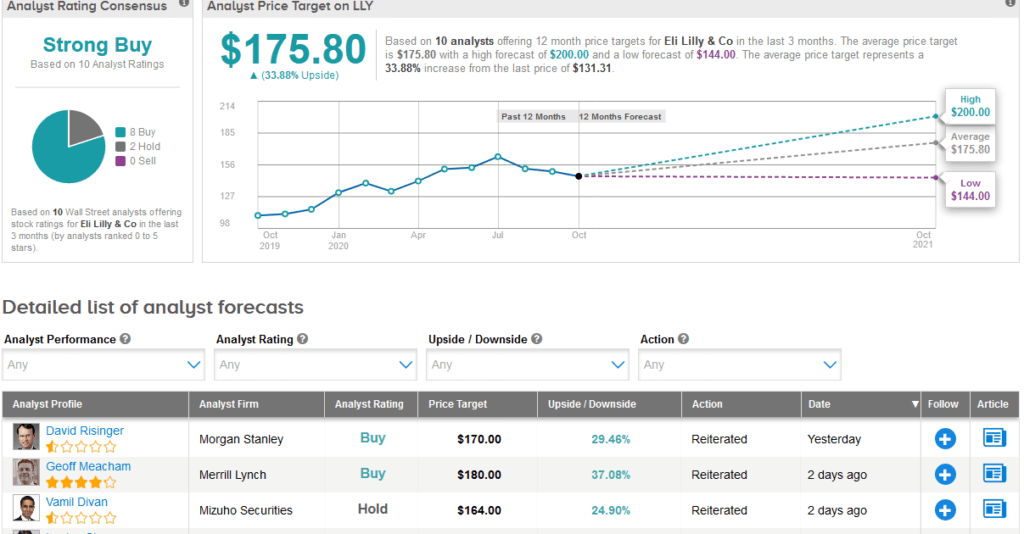
Following LLY’s quarterly results this week, Mizuho analyst Vamil Divan reiterated a Hold rating on the stock with a $164 price target, noting that the company maintained its FY 2020 sales and EPS guidance, and commented that the higher end of the revenue range would “likely require the inclusion of moderate revenue from potential COVID-19 treatments, which is possible but not certain at this point.”
“LLY shares have faced some pressure since the company announced 2Q 2020 results three months ago, and we would expect further pressure given the disappointing 3Q 2020 results,” Divan wrote in a note to investors.
Overall, the stock scores a bullish Strong Buy Street consensus. That’s with a $175.80 average analyst price target, indicating 34% upside potential lies ahead.
Related News:
Gilead Cuts 2020 Guidance As Remdesivir Sales Disappoint
Eli Lilly Ends Covid-19 Trial In Hospitalized Patients On Disappointing Data
Dexcom Drops 9% As Revenue Beat Fails To Impress Investors