Eli Lilly said it submitted an initial request to the US Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of its LY-CoV555 monotherapy in higher-risk patients who have been recently diagnosed with mild-to-moderate COVID-19. Shares rose 1.9% in Wednesday’s extended trading session after closing 3.6% higher.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Eli Lilly (LLY) disclosed that trial results showed that its developmental antibody treatment could be effective for COVID-19 patients “with a high risk for serious outcomes.” The drugmaker added said that the initial analysis of its Phase 2 clinical trial demonstrated that combination therapy of its LY-CoV555 and LY-CoV016 antibodies significantly reduced viral load at day 11.
Daniel Skovronsky, Lilly’s chief scientific officer said that “We believe the data generated to date provide sufficient evidence that both monotherapy and combination therapy may be effective to treat COVID-19 in patients with a high risk for serious outcomes. Lilly is diligently working with regulators around the world to make these treatments available.” (See LLY stock analysis on TipRanks).
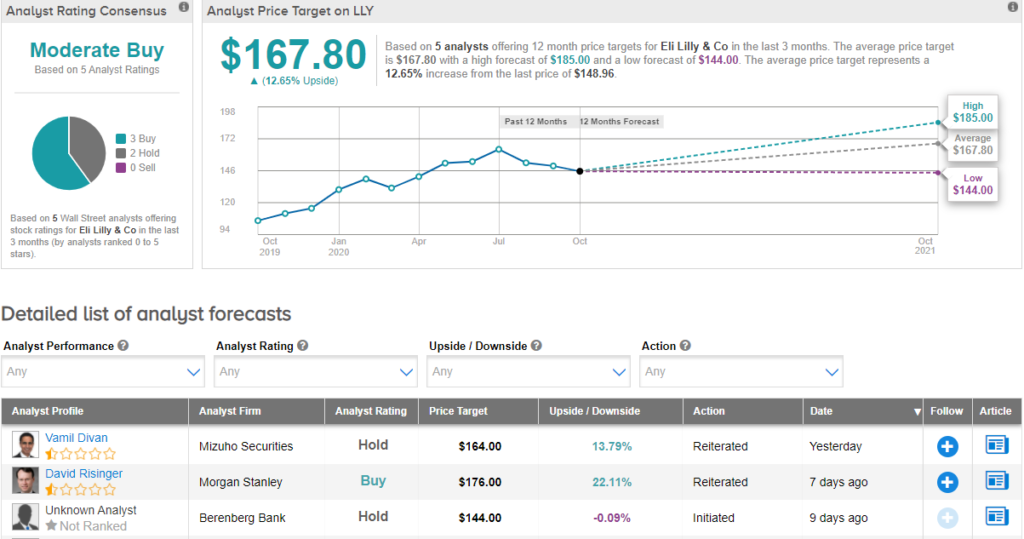
Following the company’s announcement, Mizuho Securities analyst Vamil Divan reiterated his Hold rating and a price target of $164 (10.1% upside potential). In a note to investors, Divan said that “We wonder where the combination would fall in the COVID treatment algorithm, how/where patients would receive it, and how Lilly may price the therapy, but overall find today’s data release encouraging.”
Currently, the Street has a cautiously optimistic outlook on the stock. The Moderate Buy analyst consensus is based on 3 Buys and 2 Holds. With shares up over 13% year-to-date, the average price target of $167.80 implies further upside potential of about 12.7% to current levels.
Related News:
Vir Biotechnology Kicks Off Covid-19 Treatment Trial; Shares Rise 6.1%
ChromaDex Pops 28% On Anti-Ageing Supplement Covid-19 Study
Pfizer-BioNTech Covid-19 Candidate Fast-Tracked By EU Agency; Shares Rise









