Germany’s CureVac announced that it will set up a European network to boost the manufacturing capacity of its COVID-19 vaccine candidate to up to 300 million doses in 2021 and up to 600 million doses in 2022.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Specifically, CureVac (CVAC) plans to build a broad and integrated European vaccine manufacturing network consisting of experienced CDMO (Contract Development and Manufacturing Organization) partners for each of the key steps involved in the manufacturing of its mRNA-based CVnCoV vaccine candidate. The manufacturing network will leverage expertise and capacity across Germany, France, the Netherlands, Belgium, Spain and Austria, as well as potentially Sweden, Poland, Italy and Ireland.
CureVac is on track to initiate the pivotal Phase 2b/3 clinical study of its vaccine candidate before the end of 2020. The Phase 1 interim data released earlier this month showed that CVnCoV was generally well tolerated and induced strong antibody responses in addition to first indication of T cell activation. The quality of immune response was found to be comparable to recovered COVID-19 patients, closely mimicking the immune response after natural COVID-19 infection, the company said.
“It is our goal to ramp up the production capacity of our vaccine candidate within a short period of time to ensure a stable supply,” said CureVac’s Chief Production Officer Florian von der Mülbe. “We are currently working with experienced CDMOs across Europe to establish a solid production network. Geographic proximity is an important factor for facilitating alignment and technology transfers.”
CureVac said it expects to ink key CDMO and supplier partnerships over the coming weeks. An additional large-scale production facility supported by the European Investment Bank (EIB) at CureVac´s headquarters in Tübingen is currently in development, the company said.
The announcement comes a day after CureVac sealed an agreement with the European Commission for the supply of 405 million doses of its COVID-19 vaccine candidate. The contract will provide European Union member states with up to 225 million doses of the vaccine and includes the option for an additional purchase of 180 million doses. The mRNA vaccine will be supplied once it has been proven to be safe and effective against COVID-19.
Under the terms of the agreement, CureVac will receive an upfront payment to support the advanced clinical development of CVnCoV and the current ramp-up of its manufacturing network.
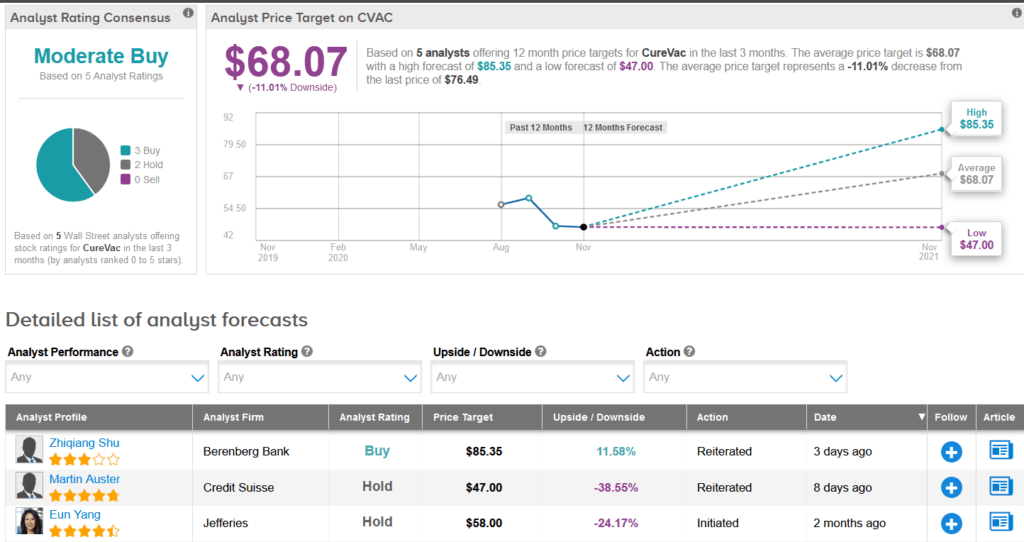
Shares of CureVac have jumped 43% over the past month. Meanwhile, Berenberg Bank analyst Zhiqiang Shu on Nov. 11 raised the stock’s price target to $72 from $68 and reiterated a Buy rating.
“We think Phase 1 results of its COVID-19 vaccine are encouraging and suggest a differentiated profile,” Shu commented in a note to investors. “The positive Phase 3 outcome from another mRNA-based COVID-19 vaccine increases our confidence of the mRNA approach.” (See CVAC stock analysis on TipRanks)
The rest of the Street is cautiously optimistic on the stock. The Moderate Buy analyst consensus shows 3 Buys versus 2 Holds. The average price target stands at $68.07, indicating downside potential of 11% over the coming 12 months.
Related News:
Pfizer Kicks Off Covid-19 Vaccine Pilot Delivery Program In US – Report
BioNTech, Fosun Pharma Get Green Light To Start Covid-19 Vaccine Trial In China
Moderna Says Covid-19 Vaccine Candidate 94.5% Effective; Shares Pop 15%