Shares in Chembio Diagnostics (CEMI) rose 12% in Monday’s after-hours trading, after the company announced a new $628,071 contract from the Biomedical Advanced Research and Development Authority (BARDA)- part of the U.S. Department of Health and Human Services.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Chembio will use the funds to accelerate development of its DPP COVID-19 Antigen System, which is expected to consist of a DPP COVID-19 Antigen Assay and DPP Micro Reader and to use a respiratory specimen, such as a nasal or nasopharyngeal swab, to detect SARS-CoV-2 antigens.
According to the statement, the contract is intended to assist Chembio in developing a COVID-19 point-of-care antigen system using Chembio’s DPP technology and requesting a U.S. Food and Drug Administration (FDA) emergency use authorization for the system. The award is to be distributed in periodic funding over the next several months.
“We are honored to again partner with BARDA and appreciate their support on a shared mission to expand and decentralize COVID-19 testing,” stated Rick Eberly, Chembio’s President and Chief Executive Officer. “We believe offering virus detection for diagnosis at the point of care can improve clinical outcomes and play a major role in combating the ongoing pandemic.”
Shares in CEMI are down 12% year-to-date, after the FDA revoked emergency use authorization for Chembio’s DPP COVID-19 IgM/IgG antibody test due to concerns over the accuracy of the test.
“While the FDA’s revocation of CEMI’s EUA for its serology test comes as a surprise, it serves as a humble reminder that execution and transparency risks are magnified for micro-cap companies” commented Canaccord Genuity analyst Max Masucci on June 17.
He subsequently downgraded his CEMI rating to hold while lowering his price target from $22 to $7, before terminating coverage altogether. “Our prior Buy thesis had been largely underpinned by the prospects for the company’s DPP COVID-19 antibody test, but that thesis is no longer intact after the FDA revoked emergency use authorization for CEMI’s serology test” the analyst explained.
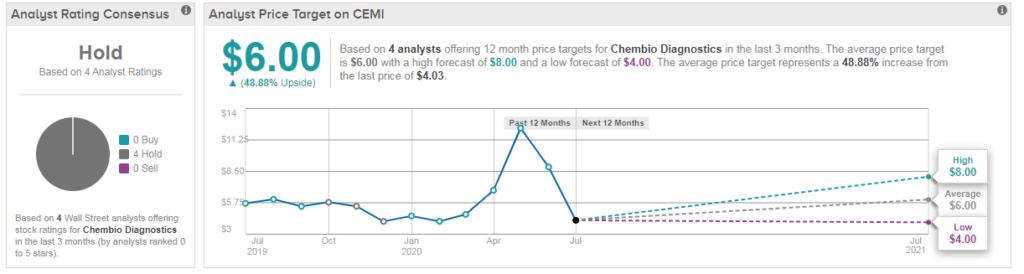
Indeed, four other analysts also downgraded the stock following the FDA update, giving Chembio a Hold consensus. The average analyst price target of $6 translates into 48% upside potential from current levels. (See CEMI stock analysis on TipRanks)
Related News:
CytoDyn Signs Distribution Deal For Covid-19 Treatment Leronlimab
Gilead’s Covid-19 Remdesivir Therapy Gets Conditional European Nod
ObsEva Plunges 47%- But Gains After-Hours- On Uterine Fibroid Data