BioNTech and Shanghai partner Fosun Pharma have been granted regulatory approval to commence the clinical trial for their mRNA-based COVID-19 vaccine candidate, BNT162b2, in mainland China. BioNTech is up 2.7% in Tuesday’s pre-market trading session.
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
Following the approval by the China National Medical Products Administration, BioNTech (BNTX), through its partner Fosun Pharma, will now kick off a Phase 2 clinical trial in mainland China. Earlier this year, BioNTech and Fosun Pharma announced a strategic collaboration to work jointly on the development and commercialization of potential COVID-19 vaccine products based on BioNTech’s mRNA technology platform in mainland China, Hong Kong, as well as in the Macau and Taiwan regions.
The BNT162b2 vaccine candidate is based on BioNTech’s proprietary mRNA technology and supported by Pfizer’s (PFE) global vaccine development and manufacturing capabilities. It encodes an optimized SARS-CoV-2 full-length spike glycoprotein (S), which is a target of virus neutralizing antibodies. Earlier this month, BioNTech and Pfizer published first interim data from a global Phase 3 clinical study suggesting the mRNA-based vaccine candidate is more than 90% effective in preventing COVID-19 in subjects who had no evidence of a previous infection.
“This start of the b2 trial in China, in conjunction with the recent interim analysis of the global Phase 3 trial that indicates that our lead candidate may be effective in protecting against COVID19, is another important step forward,” said BioNTech CEO Ugur Sahin. “Time is of the essence in this effort and we greatly appreciate the support by Chinese regulators and the collaboration with our Chinese partner Fosun Pharma. We will continue to collaborate closely to advance clinical development in China toward market approval.”
BNT162b2 is currently in Phase 3 clinical trials in the US, Germany, Argentina, Brazil, South Africa, Turkey and other countries, and received fast track designation from the US Food and Drug Administration. In addition, a rolling submission to the European Medicines Agency (EMA) for BNT162b2 has also been initiated.
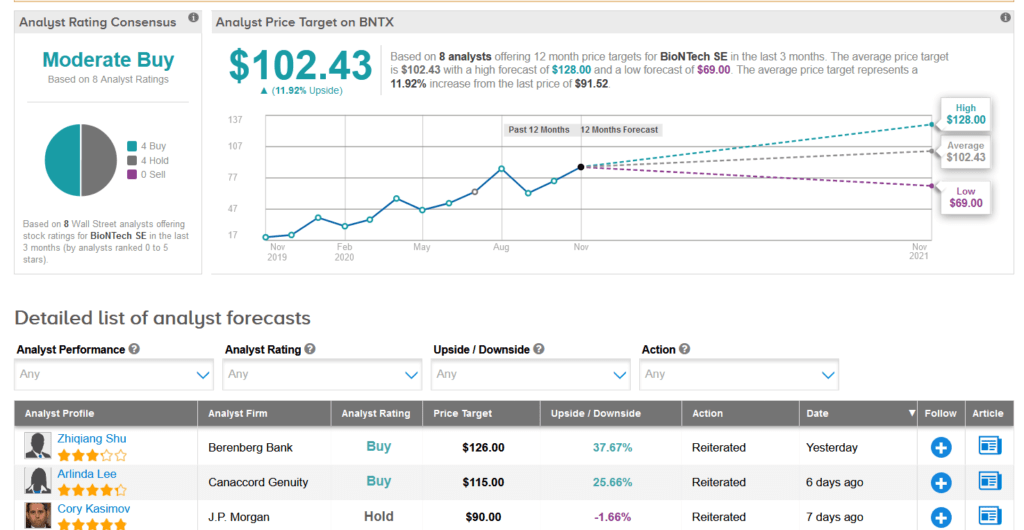
With BioNTech shares up a whopping 170% so far this year, analysts now forecast another 12% upside potential lies ahead over the coming year, setting the average price target at $102.43.
In reaction to the positive results from the vaccine candidate’s global Phase 3 study, Berenberg analyst Zhiqiang Shu ramped up the stock’s price target to $126 from $98 and reiterated a Buy rating.
“We think Phase 3 preliminary results position BNT162 as a highly competitive COVID-19 vaccine and point to a strong commercial case for the company,” Shu commented in a note to investors. “We think this validates BNTX’s mRNA technology, which is on track to deliver multiple cancer treatments.”
The rest of the Street is cautiously optimistic on the stock with a Moderate Buy analyst consensus, which is divided between 4 Buys and 4 Holds. (See BioNTech stock analysis on TipRanks).
Related News:
Moderna Says Covid-19 Vaccine Candidate 94.5% Effective; Shares Pop 15%
J&J Kicks Off Two-Dose Phase 3 Covid-19 Trial In UK; Street Bullish
Pfizer, BioNTech Announce COVID-19 Vaccine is 90% Effective









