Becton Dickinson and Co. announced that its rapid, point-of-care, SARS-CoV-2 antigen test has been granted European regulatory approval, sending its shares up 3.7%.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
BD (BDX) said that it received the regulatory CE mark to launch the test in Europe. As a result, the US medical device maker expects the tests to be commercially available at the end of October for countries in Europe that recognize the CE mark. BD has been ramping up its global manufacturing network to produce 8 million SARS-CoV-2 antigen tests per month by October and expects to produce 12 million tests per month by March 2021.
The Covid-19 detection test, which runs on BD’s Veritor Plus system provides real-time results in 15 minutes on the easy-to-use, portable instrument, while the patient is still onsite.
“Availability of the SARS-CoV-2 assay on the BD Veritor Plus system in Europe builds on our molecular test on the BD MAX system that has been available since March,” said Roland Goette, president of BD EMEA Region. “The addition of a truly portable, point-of-care test that can deliver results while the patient waits will be welcomed by health care providers and patients alike to help protect against additional waves of COVID-19.”
The detection test has been available in the US since July after receiving Emergency Use Authorization by the US Food and Drug Administration (FDA).
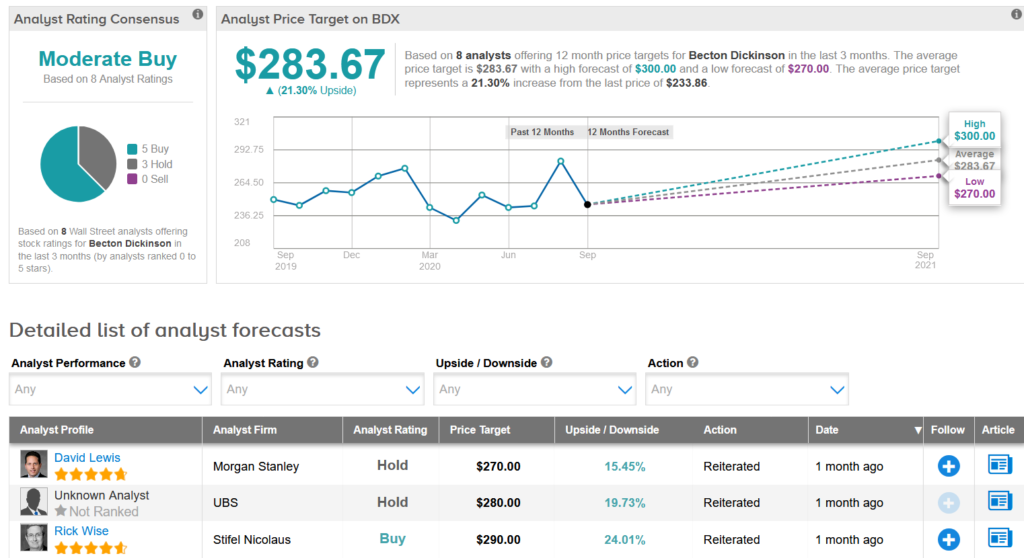
Shares in BDX are currently down 14% year-to-date, and analysts have a cautiously optimistic take on the stock’s outlook with a Moderate Buy consensus. Meanwhile the average analyst price target of $283.67 indicates 21% upside potential lies ahead.
Raymond James analyst Lawrence Keusch recently raised the stock’s price target to $285 from $265 and maintained a Buy rating, saying that he expects significant benefits from the company’s SARS-CoV-2 testing platforms in FY21.
In addition, Keusch forecasts incremental needle/syringe revenue from vaccine manufacturers driving sales growth of 11%. (See BDX stock analysis on TipRanks).
Related News:
Regeneron Up 4% As Covid-19 Cocktail Lowers Viral Levels; Analyst Says Hold
InflaRx Pops 6% On ‘Promising’ Data From Covid-19 Therapeutic Trial
Coca-Cola, Molson Coors Team Up For US Topo Chico Alcoholic Drink