Baxter International has been granted Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA) for its Regiocit, to be used during the coronavirus pandemic as a replacement solution only in adult patients suffering from acute kidney injury.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Baxter (BAX) said that under the EUA, Regiocit is now authorized to be used in adult patients being treated with continuous renal replacement therapy (CRRT) and for whom regional citrate anticoagulation is appropriate during the COVID-19 pandemic.
Acute kidney injury, a potentially life-threatening condition where the kidneys suddenly stop working and fluid and uremic toxins build up in the body, is one of many complications affecting COVID-19 patients. CRRT mimics many of the functions of the natural kidney and is the cornerstone of treatment in patients with severe acute kidney injury. Regiocitis the only authorized citrate-based replacement solution available in the US for use in CRRT during the COVID-19 pandemic, Baxter said.
“Demand for CRRT remains elevated as the COVID-19 pandemic continues to progress, and we’re proud to offer Regiocitas an important new option to help healthcare providers in the US optimize care for critically ill patients requiring CRRT and regional citrate anticoagulation, while bringing an additional supply of replacement solutions to the US,” said Reaz Rasul, general manager of Baxter’s Acute Therapies business.
Regiocitcontains physiological concentrations of sodium (140 mmol/l), chloride (86 mmol/l) and a low concentration of citrate (18 mmol/L) and can provide the necessary pre-filter volumes required to replace filtration losses during convective therapies. It is used in combination with other standard dialysis and replacement solutions that supplement missing electrolytes such as potassium, magnesium and phosphate.
Deerfield, Illinois-based Baxter added that it continues to provide CRRT machines, fluids and sets to help healthcare facilities address patient needs around the world.
Regiocit has not been approved by the FDA in the US but is currently in use in countries around the world, including in Europe and Asia. A limited initial shipment will be available in the US immediately, with more significant production ramping up throughout the coming weeks and months, the company said.
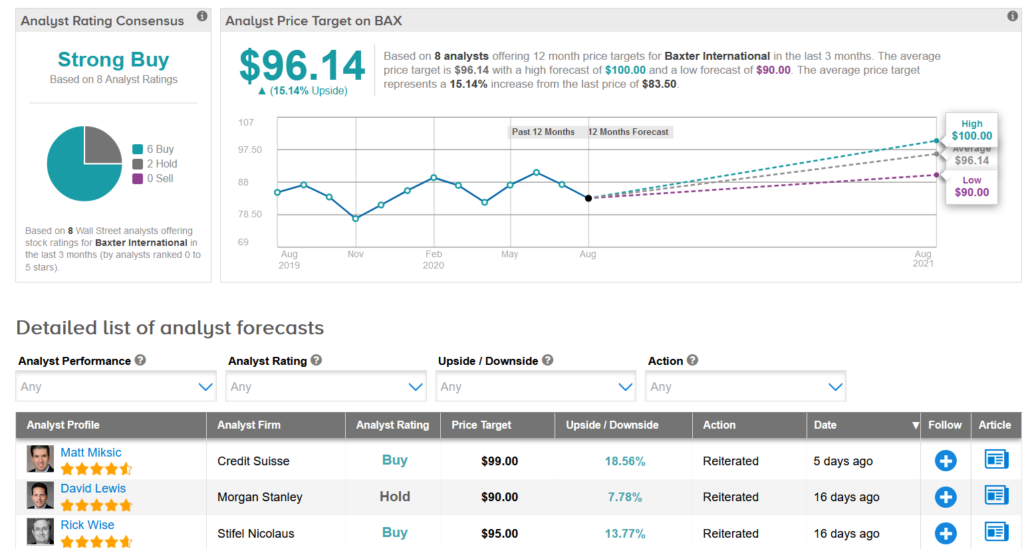
Baxter shares advanced 1.6% in Friday’s after-market session and are now trading little changed from their start of the year level. The $96.14 average analyst price target indicates 15% upside potential in the shares over the coming 12 months.
Credit Suisse analyst Matt Miksic last week reiterated a Buy rating on the stock with a $99 price target, saying that he views the stock as attractive at current levels, trading significantly below its 3-year average multiple relative to the S&P 500.
“Strength in Acute Therapies is expected to continue, driven mostly by consumable growth and utilization related to COVID-19, with some deceleration in 2H vs. 1H,” Miksic wrote in a note to investors.
Overall Wall Street analysts share Miksic’s bullish outlook. The Strong Buy consensus boasts 6 Buys versus 3 Holds. (See Baxter stock analysis on TipRanks)
Related News:
Novavax Pops 7% On South Korea Deal For Global Covid-19 Vaccine Supply
EU Secures 200M Doses Of Johnson & Johnson’s Covid-19 Vaccine Candidate
Quidel’s Lyra Covid-19 Test Gets Nod For Sale In Canada; Shares Rise 6%









