AstraZeneca announced that it has started enrolling adults for a Phase 3 clinical trial in the US of its Covid-19 vaccine candidate AZD1222.
AstraZeneca (AZN) said that the trial, which will include 30,000 participants over the age of 18, will assess the safety, efficacy and immunogenicity of AZD1222 for the prevention of Covid-19.
The US trial, called D8110C00001, is funded by the Biomedical Advanced Development Authority (BARDA) and the National Institute of Allergy and Infectious Diseases (NIAID), and led by AstraZeneca.
“We are pleased that AZD1222 demonstrated safety and immunogenicity across all adult age groups and are proud to be collaborating with BARDA and NIAID to accelerate the development of this vaccine,” AstraZeneca’s Mene Pangalos said. “Should clinical trials demonstrate the vaccine protects against COVID-19 disease and is approved for use, we will work hard to make it globally available in a fair and equitable manner as rapidly as possible.”
Participants in the trial will receive two doses of either AZD1222 or a saline control, four weeks apart, with twice as many participants receiving the potential vaccine than the saline control. The trial is assessing efficacy and safety of the vaccine in all participants, while local and systemic reactions and immune responses will be assessed in 3,000 participants.
AstraZeneca added that clinical development of AZD1222 is progressing globally with late-stage clinical trials ongoing in the UK, Brazil and South Africa and trials are planned to start in Japan and Russia.
These trials, together with the US Phase III clinical trial will enrol up to 50,000 participants globally. Results from the late-stage trials are anticipated later this year, depending on the rate of infection within the clinical trial communities.
In July, AstraZeneca reported that interim results from the ongoing Phase I/II COV001 trial showed that AZD1222 was tolerated and generated robust immune responses against the SARS-CoV-2 virus in all evaluated participants.
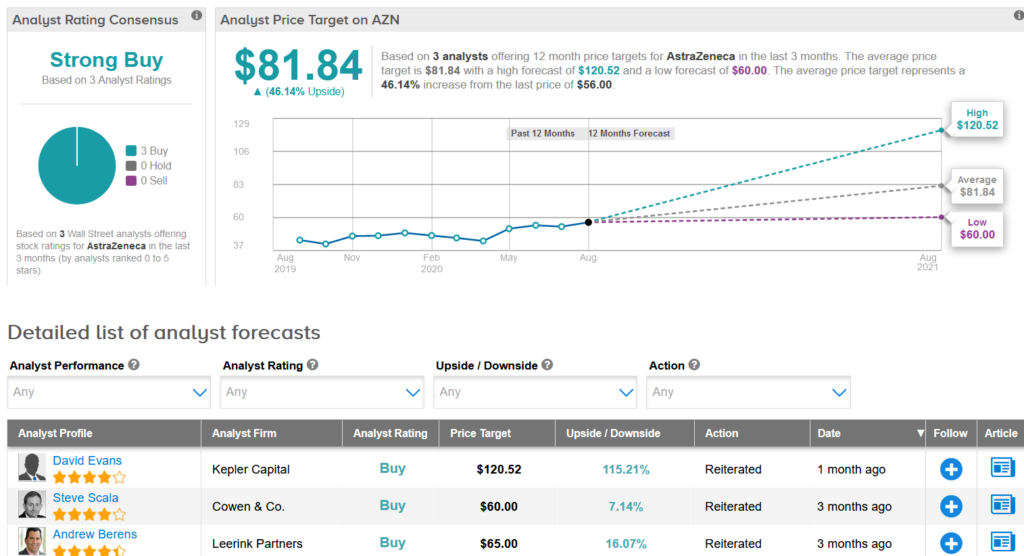
AZN shares have gained 12% this year as the drugmaker joined the list of companies engaged in the development of a potential coronavirus vaccine. Looking ahead, the $81.84 average analyst price target puts the upside potential at a promising 46% in the coming 12 months.
Overall, the stock scores a Strong Buy consensus from the analyst community based on 3 unanimous Buy ratings. (See AstraZeneca stock analysis on TipRanks).
Related News:
T2 Bioystems Spikes 19% On FDA Nod For Covid-19 Molecular Test
Moderna In Talks To Supply 40M Doses Of Its Covid-19 Vaccine To Japan
AstraZeneca Rises On Report Trump Could Fast-Track Covid-19 Vaccine Candidate
















