AstraZeneca (AZN) has agreed to supply up to 400 million doses of the University of Oxford’s COVID-19 vaccine, AZD1222, to Europe’s Inclusive Vaccines Alliance (IVA), with deliveries starting by the end of 2020.
Total manufacturing capacity for AZD1222 now stands at two billion doses, says AZN.
Spearheaded by Germany, France, Italy and the Netherlands, the IVA says it aims to accelerate the supply of the vaccine and to make it available to other European countries that wish to participate in the initiative.
At the same time, UK-based AstraZeneca continues to build several global supply chains, including for Europe. “The company is seeking to expand manufacturing capacity further and is open to collaborating with other companies in order to meet its commitment to support access to the vaccine at no profit during the pandemic” AZN states.
Pascal Soriot, CEO of AstraZeneca, commented: “With our European supply chain due to begin production soon, we hope to make the vaccine available widely and rapidly.”
Indeed, the company recently entered similar agreements with the UK, US, the Coalition for Epidemic Preparedness Innovations and Gavi the Vaccine Alliance for 700 million doses. It has also agreed a license with the Serum Institute of India for the supply of an additional one billion doses for low and middle-income countries.
Oxford University last month announced the start of a Phase II/III UK trial of AZD1222 in about 10,000 adult volunteers. Other late-stage trials are due to begin in a number of countries. AstraZeneca recognizes that the vaccine may not work but is nonetheless progressing the clinical program with speed and scaling up manufacturing at risk.
AZD1222 uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus that causes infections in chimpanzees and contains the genetic material of SARS-CoV-2 spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack COVID-19 if it later infects the body.
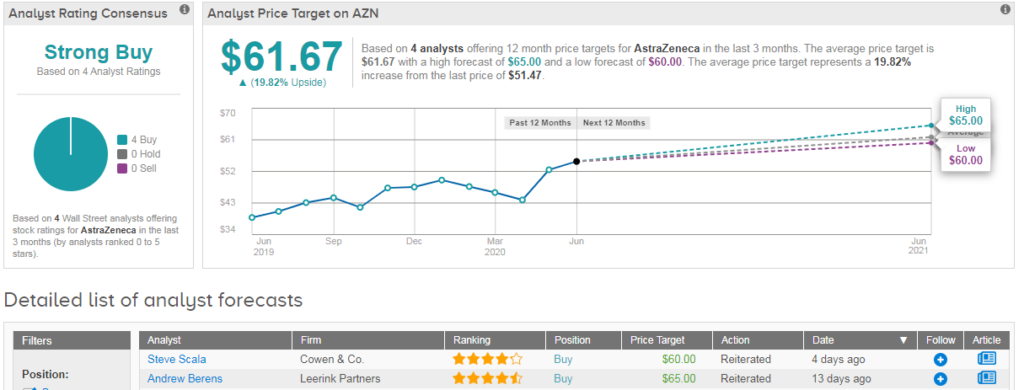
Shares in AstraZeneca are up 3% year-to-date, and analysts are, on average, predicting that 20% further upside potential lies ahead. This comes with a Strong Buy consensus based on 4 recent buy ratings. (See AstraZeneca stock analysis on TipRanks).
Related News:
Oxford Biomedica Clinches Manufacturing Deal For AstraZeneca’s Covid-19 Vaccine
5 Promising Covid-19 Vaccines Picked For Trump’s Operation Warp Speed
What Would a Merger Mean for Gilead? Top Analyst Weighs In
















