Shares of AlloVir jumped 13.6% on Friday after the US Food and Drug Administration (FDA) granted clinical trial approval for its ALVR109, an allogeneic, off-the-shelf virus-specific T cell Covid-19 therapy against SARS-CoV-2. The therapy is being designed and developed to halt the progression of COVID-19.
AlloVir (ALVR) said that following the submission of the Investigational New Drug application (IND) for its ALVR109, the FDA had imposed a clinical hold on trials citing concerns over the raw materials used in the manufacturing process. Earlier this year, the company announced that it had expanded its research partnership with Baylor College of Medicine (BCM) to “discover and develop allogeneic, off-the-shelf, virus-specific T-cell therapies for COVID-19.”
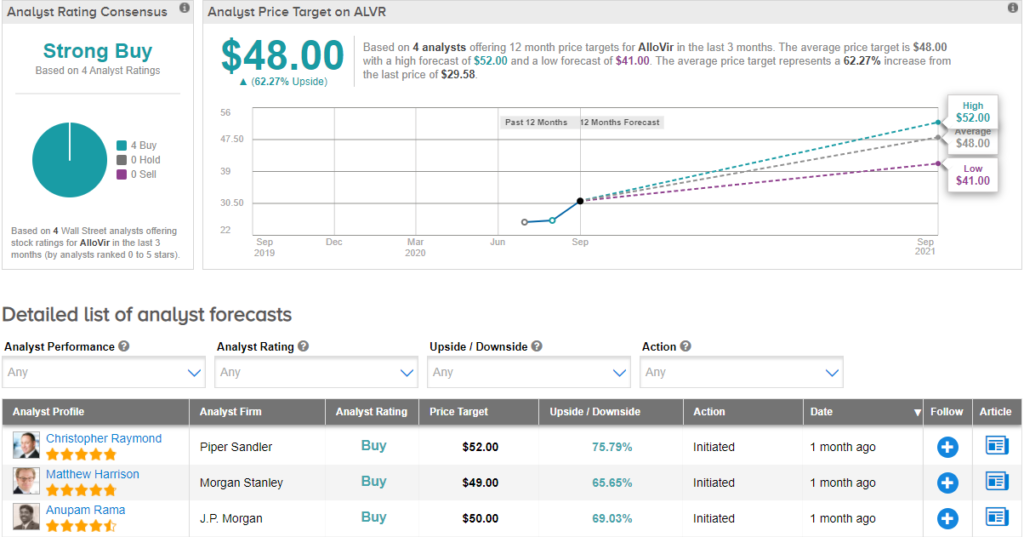
On August 24, Morgan Stanley analyst Matthew Harrison initiated coverage on the stock with a Buy rating and price target of $49 (65.7% upside potential) citing a multi-billion opportunity in the T-cell therapy. Harrison said, “Broad T-cell platform provides significant cell therapy opportunity which can drive $1B+ in peak sales. Lead program Viralym-M is already derisked with encouraging PhII data. Respiratory diseases and COVID offer upside.” (See ALVR stock analysis on TipRanks).
Currently, the Street has a bullish outlook on the stock, with a Strong Buy analyst consensus. With shares up nearly 16.5% since its listing on July 30, the average analyst price target of $48 implies further upside potential of 62.3% from current levels.
Related News:
Pfizer Reveals Efficacy Criteria For Key Covid-19 Vaccine Trials
Sanofi, Regeneron’s Libtayo Delivers Positive Results In Pivotal Lung Cancer Trial
Eli Lilly’s Verzenio Treatment Lowers Breast Cancer Recurrence By 25%
















