Abiomed (ABMD) has announced that the US Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for left-sided Impella heart pumps to provide left ventricular unloading and support to COVID-19 patients who are undergoing ECMO treatment and develop pulmonary edema or myocarditis.
Extracorporeal membrane oxygenation (ECMO) is a treatment that uses a pump to circulate blood through an artificial lung back into the bloodstream.
COVID-19 causes widespread inflammation which can result in damage to the lungs and heart. This damage may cause severe left ventricular dysfunction manifesting as pulmonary edema and/or myocarditis.
According to Abiomed, Impella combined with ECMO therapy has become an important tool for physicians treating COVID-19 patients suffering from both heart and lung failure.
This is the second EUA the FDA has granted for Impella during the COVID-19 pandemic. On May 29, the FDA issued an EUA to expand the use of Impella RP to include patients suffering from COVID-19-related right ventricular complications, including right ventricular dysfunction associated with pulmonary embolism.
To date, Impella is the only cardiovascular therapeutic device that has received FDA emergency use authorization to treat COVID-19 patients.
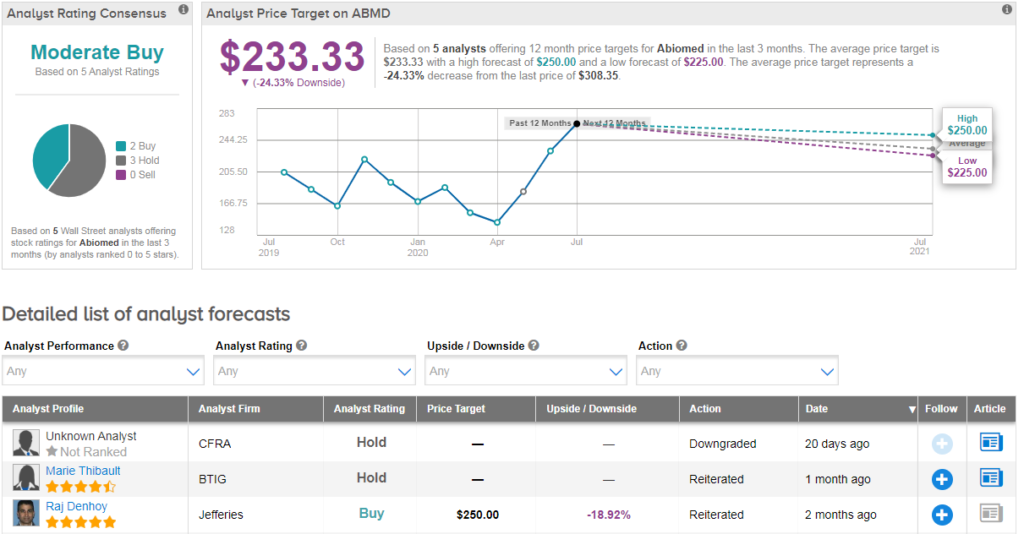
Shares in Abiomed are rising 2% in Tuesday’s pre-market trading. Year-to-date the stock has soared over 80%, and analysts have a cautiously optimistic Moderate Buy consensus on ABMD’s outlook. Due to the recent rally, the average analyst price target indicates that shares could pull back 24% from current levels.
For instance, BTIG analyst Marie Thibault has a hold rating on the stock writing “We remain at Neutral due to valuation.” She notes that during the pandemic, ABMD has been emphasizing its education and training efforts which may help boost Impella utilization once the pandemic reaction passes.
“Layering on additional clinical trial work, pipeline products, and the potential for M&A, we believe Abiomed is poised to gradually return to strong sustainable growth” she tells investors.
Ultimately however Thibault believes this that much of this optimism is already priced in. “ABMD’s share price has risen alongside much of MedTech and the stock trades at a premium EV/Sales multiple versus the fast-growing comp group average” the analyst concludes. (See ABMD stock analysis on TipRanks).
Related News:
Merck, Novocure Team Up On Lung Cancer Treatment; Trial Set For 2H20
AstraZeneca-Merck Pancreatic Cancer Drug Wins European Approval
Regeneron: Covid-19 Antibody Combo Prevents & Treats Disease In Animals
















