Abbott Laboratories (ABT) is now holding trials to try and expand its rapid Covid-19 test to people who show no symptoms of the virus, ABT’s CEO Robert Ford has revealed to CNBC.
“We are working on developing data for asymptomatic claims, so we are running our clinical trial, and we’ll eventually have data to be able to support that,” Ford told CNBC.
“In the meantime, we see it being able to be deployed, because it doesn’t requirement an instrument … through the federal government, through the state governments, through schools, through employers, or even through retail clinics,” the CEO continued. “We think that’s a great opportunity to be able to line up this volume.”
The Abbott BinaxNOW COVID-19 Ag Card has just received emergency use authorization from the US Food and Drug Administration (FDA), does not require instrumentation, and can deliver Covid-19 test results in 15 minutes or less.
It uses nasal swabs and is simple to use, inexpensive, and can be easily employed by medical personnel or trained operators in certain non-clinical environments operating through a CLIA certificate.
However, as it currently stands, the test is only approved for individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset.
Earlier this week the US government awarded a $760 million contract to Abbott for the delivery of 150 million rapid BinaxNOW Point of Care (POC) Covid-19 tests.
“The introduction of Abbott’s antigen test is another incredibly valuable result of President Trump’s all-of-America approach to constructing our world-leading COVID-19 testing capacity,” said HHS Secretary Alex Azar.
“By strategically distributing 150 million of these tests to where they’re needed most, we can track the virus like never before and protect millions of Americans at risk in especially vulnerable situations.”
Abbott says it has the capability to scale up to meet the demand for antigen testing across the country and is the only known source that can immediately provide the required items to meet HHS’s urgent needs. (See Abbott stock analysis on TipRanks)
“Early data for ABT’s test demonstrated sensitivity and specificity of 97.1%/98.5% when used within the first seven days of symptom onset. Abbott plans to ship “tens of millions” of tests in September and expects to ramp to 50M tests/month by the beginning of October” commented Canaccord Genuity’s Max Masucci on the news.
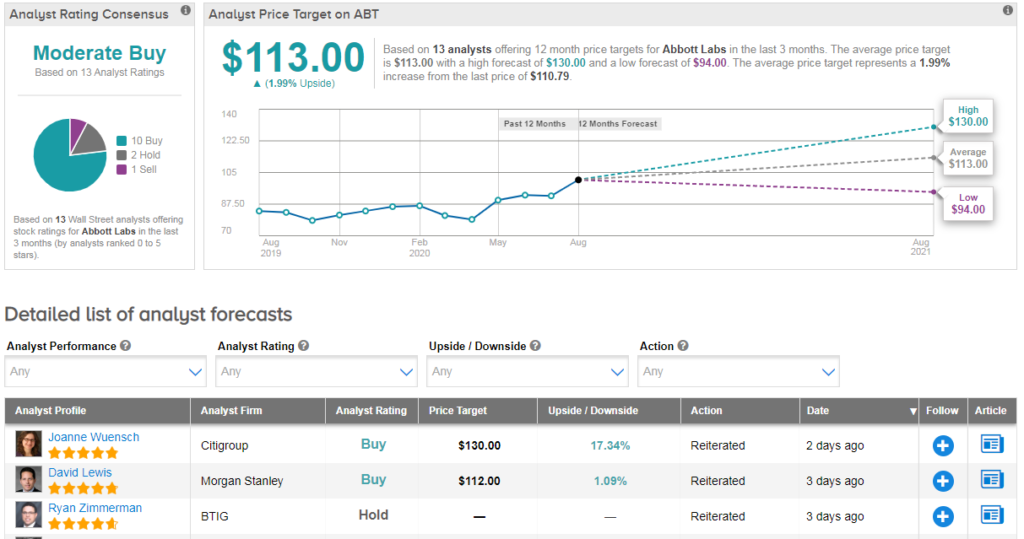
Shares in ABT are now trading up 27% year-to-date, with an 8% spike over the last five days following the FDA approval. The stock has a cautiously optimistic Moderate Buy Street consensus with 10 recent buy ratings, 2 holds and 1 sell. Meanwhile the average analyst price target of $113 shows upside potential of just 2%.
Related News:
Moderna Pops 6% On Covid-19 Vaccine Response In Older Adults; Analyst Says Buy Now
AstraZeneca Rises On Report Trump Could Fast-Track Covid-19 Vaccine Candidate
Abbott Jumps 22% On Emergency Use Nod For Credit Card Size Covid-19 Test
















