A class action lawsuit was filed against Sage Therapeutics (SAGE) by Levi & Korsinsky on August 28, 2024. The plaintiffs (shareholders) alleged that they bought SAGE stock at artificially inflated prices between April 12, 2021 and July 23, 2024 (Class Period) and are now seeking compensation for their financial losses. Investors who bought Sage Therapeutics stock during that period can click here to learn about joining the lawsuit.
Don't Miss our Black Friday Offers:
- Unlock your investing potential with TipRanks Premium - Now At 40% OFF!
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
Sage Therapeutics is a biopharmaceutical company that is focused on developing brain health medicines. The company boasts that it has developed the only two U.S. Food and Drug Administration (FDA)-approved treatments for postpartum depression (PPD).
Sage Therapeutics’ claims about the approval process for zuranolone (SAGE-217/BIIB125), its neuroactive steroid for the treatment of PPD and major depressive disorder (MDD), which is being developed in collaboration with Biogen Inc. (BIIB), and issues related to other treatments are at the heart of this lawsuit.
SAGE’s Misleading Claims
According to the lawsuit, Sage Therapeutics and two of its senior officers (Individual Defendants) are accused of deceiving investors by lying and withholding crucial information about the company’s business and prospects during the Class Period. Particularly, the Defendants are accused of omitting truthful information about zuranolone’s efficacy in treating MDD and ancillary issues from SEC filings and related material. Moreover, the company allegedly overstated the effectiveness of its SAGE-324 treatment for essential tremor (ET).
For instance, in a press release issued at the beginning of the Class Period, SAGE’s CEO said that the company exceeded the high bar set in the KINETIC Study and that SAGE-324 met the primary endpoint in the trial. The CEO also said that the data presented in the press release supported the continued development of SAGE-324.
Further, in a press release on October 19, 2021, the company’s CEO stated that in the pre-NDA (New Drug Application) meeting, the FDA’s response on the regulatory pathway for zuranolone remained consistent with previous discussions and that zuranolone displayed “remarkably consistent, rapid, and sustained reductions in depressive symptoms, including anxiety and sleep loss.”
In contrast to these disclosures, subsequent events (discussed below) revealed that the company allegedly overstated its business prospects.
Plaintiffs’ Arguments
The plaintiffs maintain that the Defendants made false and misleading public statements throughout the Class Period.
The truth came out in multiple events that occurred between August 4, 2023 and July 24, 2024. Mainly, in the press release dated July 24, 2024, the company stated that the Phase 2 study of SAGE-324 failed to demonstrate a “statistically significant dose-response relationship in change from baseline to Day 91 based on the primary endpoint” in participants with ET. Given this disappointment, the company and Biogen closed the open label safety study of SAGE-324 in ET and decided not to conduct further clinical development of SAGE-324 in ET.
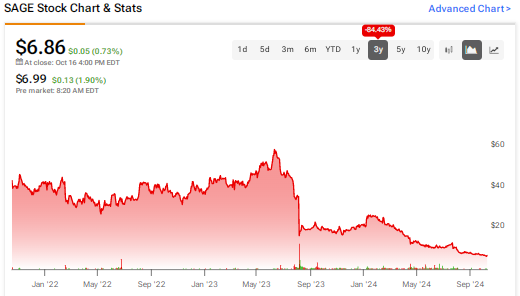
Previously, the company faced a setback in August 2023, when the FDA approved the NDA for zuranolone as a treatment for adults with PPD but issued a Complete Response Letter (CRL) for the NDA for zuranolone as a treatment for MDD. The FDA explained that the company’s application did not provide enough evidence to establish the effectiveness of zuranolone for the treatment of MDD. SAGE stock plunged about 54% on August 7, 2023 in reaction to this news.
Additionally, in April 2024, the company announced that its Phase 2 study of the investigational oral medicine dalzanemdor (SAGE-718) for the treatment of mild cognitive impairment (MCI) in Parkinson’s Disease (PD) did not meet its primary endpoint. Consequently, the company decided not to pursue any further development of dalzanemdor in Parkinson’s Disease.
To conclude, Sage Therapeutics allegedly misled investors about the prospects of its business. Consequently, SAGE stock has declined over 84% in the past three years, causing damage to shareholder returns.