Shares of Cidara Therapeutics (NASDAQ: CDTX) fell in pre-market trading on Thursday even as the company developing long-acting therapeutics announced along with Melinta Therapeutics that the U.S. Food and Drug Administration (FDA) had approved REZZAYO (rezafungin for injection) for the treatment of candidemia and invasive candidiasis in adults with limited or no alternative treatment options. Candidemia and invasive candidiasis are infections caused by yeast or a type of fungus called Candida.
Rezzayo is the U.S. FDA’s first approved echinocandin over the past ten years.
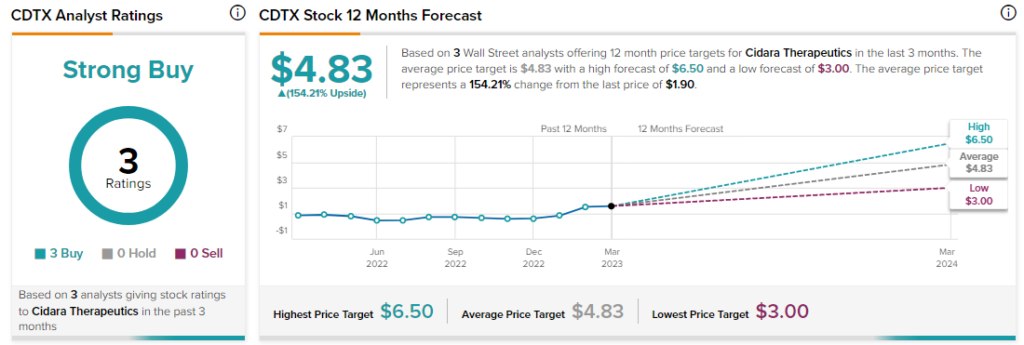
Analysts rate CDTX stock a Strong Buy with a unanimous three Buys.
Questions or Comments about the article? Write to editor@tipranks.com
















