Biotech stocks like Cabaletta Bio (NASDAQ:CABA) tend to live or die by FDA approval. This makes sense; that’s the only way their products get to market. After news emerged about Cabaletta’s latest FDA application, it shot up over 14% in Tuesday’s trading.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Cabaletta Bio revealed that its Investigational New Drug application at the FED had been cleared, which means that it could start up Phase 1 / 2 trials for its latest drug, CABA-201. CABA-201 focuses on treating muscular disorder myositis, a condition that sees elements of the immune system attacking muscle structure, breaking it down, and making movement more difficult. While there isn’t a cure—and CABA-201 doesn’t seem to be a possible one—muscular disorder myositis can be put into remission, which makes movement easier for those suffering from the disease.
Cabaletta has a complete testing plan all ready to go, running separate tests for patients with various kinds of myositis. This is actually the second time in the last three months that Cabaletta has landed testing approval from the FDA; previously, Cabaletta landed approval to try CABA-201 as a systemic lupus erythematosus (SLE) treatment. While it will likely be a while before we find out the news, CABA-201 turning out to be as versatile as it might be could be a big win for Cabaletta.
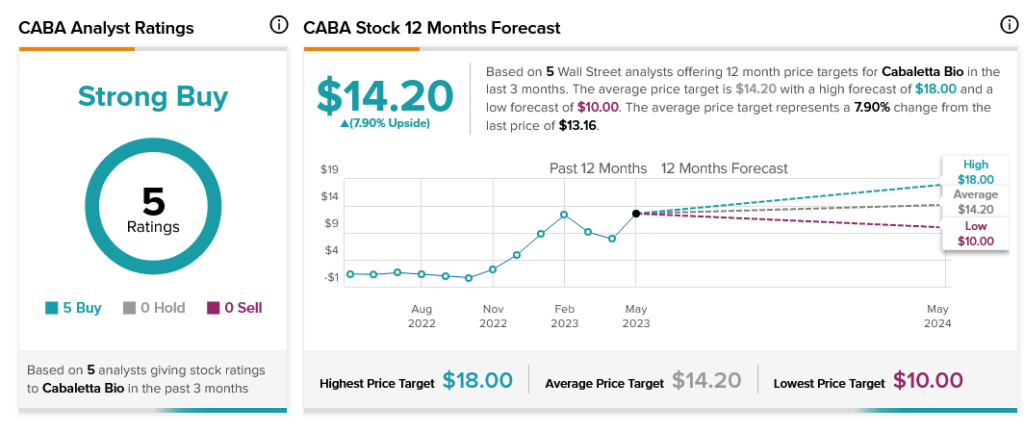
Meanwhile, analysts are all in on Cabaletta Bio, as five Buy recommendations make CABA stock a Strong Buy. However, it offers investors only 7.9% upside potential thanks to its average price target of $14.20.









