During a Late-Breaking Science session at the American Heart Association (AHA) Scientific Sessions 2021, Bristol Myers Squibb (BMY) and Janssen Pharmaceuticals, a Johnson and Johnson (JNJ) company, presented results from the Phase 2 AXIOMATIC-TKR study in patients undergoing total knee replacement (TKR) surgery.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
This study is designed to evaluate the efficacy and safety of milvexian, a first-of-its-kind oral factor XIa (FXIa) inhibitor, compared to enoxaparin.
The data demonstrated by the global biopharmaceutical company showed that investigational oral milvexian reduced the risk of postoperative venous thromboembolism (VTE) in a dose dependent manner without increasing the risk of bleeding compared with enoxaparin, the company said.
The Phase 2 milvexian program has two studies, of which the TKR study is the first one. Notably, results from the ongoing Phase 2 study of milvexian for secondary stroke prevention (AXIOMATIC-SSP) are expected to be released in the first half of 2022. (See Bristol Myers Squibb stock charts on TipRanks)
Official Comments
The SVP and Head of cardiovascular development at Bristol Myers Squibb, Roland Chen, said, “We are encouraged by the results of the milvexian TKR trial, which are consistent with our scientific understanding of the FXIa mechanism. The clear dose efficacy response without increased bleeding provides additional evidence to support our belief in the promise of milvexian. We look forward to results from our second Phase 2 trial of milvexian for secondary stroke prevention, which will add to our body of evidence for milvexian and help inform our Phase 3 development program.”
See Top Smart Score Stocks on TipRanks >>
Wall Street’s Take
Recently, Argus Research analyst David Toung downgraded Bristol Myers Squibb to Hold from Buy.
According to Toung, the company’s product, Revlimid, which contributes the largest portion to total revenues, is likely to face generic competition both in the U.S. and overseas in 2022. The analyst expects this to “weigh heavily” on the company’s revenue growth.
The rest of the Street is cautiously optimistic about the stock and has a Moderate Buy consensus rating based on 5 Buys and 3 Holds. The average Bristol Myers Squibb price target of $74.17 implies 24.4% upside potential from current levels. Shares have lost 7.6% over the past year.
Risk Analysis
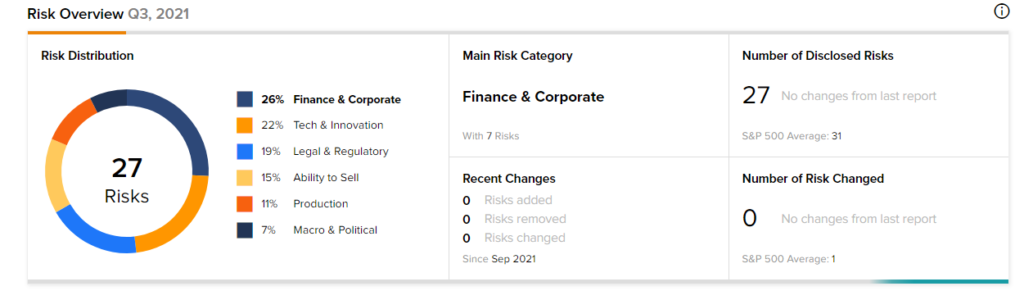
According to the new TipRanks’ Risk Factors tool, the Bristol Myers Squibb stock is at risk mainly from three factors: Finance and Corporate, Tech and Innovation, and Legal and Regulatory, which contribute 26%, 22%, and 19%, respectively, to the total 27 risks identified for the stock.
Related News:
Moderna Presents Data from Phase 1 Clinical Study of mRNA Triplet Program
Boeing to Initiate Three New Freighter Conversion Lines
Prelude Therapeutics Drops 3.3% on Quarterly Loss









