Shares of Bristol Myers Squibb (NYSE:BMY) were on an upswing in pre-market trading on Monday, following the U.S. FDA’s approval for Sotyktu (deucravacitinib). Sotyktu is an oral treatment for adults with moderate-to-severe plaque psoriasis.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Samit Hirawat, MD, Chief Medical Officer, Bristol Myers Squibb commented, “The approval of Sotyktu represents an exciting day for patients suffering from moderate-to-severe plaque psoriasis who are not satisfied with topical and conventional treatments. “
The biopharmaceutical company also presented encouraging long-term clinical efficacy data for Sotyktu.
BMY’s two-year results from the POETYK PSO long-term extension (LTE) trial indicated that out of the 262 patients who received Sotyktu, 171 had achieved Psoriasis Area and Severity Index (PASI) 75 at Week 16 of the trial. This PASI measure indicates that the severity and the psoriasis disease area were reduced by 75%.
What’s more, among these patients, the efficacy of Sotyktu was maintained for up to 112 weeks.
Is Bristol-Myers Squibb a Buy, Sell, or Hold?
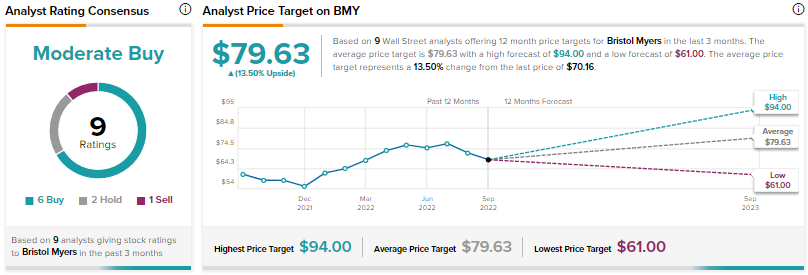
Analysts are cautiously optimistic about BMY with a Moderate Buy consensus rating based on six Buys, two Holds, and one Sell.
BMY’s average price prediction of $79.63 implies that the stock has an upside potential of around 13.5%.