BridgeBio Pharma’s stock (BBIO) jumped over 16% after the FDA approved its cardiovascular drug, Attruby. This new treatment will compete with Pfizer’s (PFE) Vyndamax, which was extremely popular in 2023. Analysts have responded positively, raising their price targets for BridgeBio after Attruby’s approval for ATTR-CM. With the company’s pipeline for late-stage development moving quickly, BBIO looks appealing in the high-risk, high-reward biopharma sector.

BridgeBio Receives a Major Boost
BridgeBio Pharma is a commercial-stage biopharmaceutical firm that focuses on developing medicines to treat genetic diseases and cancers. The company continues progressing with its drug pipeline, with three Phase 3 readouts anticipated in 2025. Recent activities include completing the screening process for the Phase 3 CALIBRATE clinical trial of encasement in ADH1 and concluding enrolment for the Phase 3 FORTIFY clinical trial for LGMD2I/R9.
BridgeBio recently received a considerable boost with FDA approval of its pipeline candidate acoramidis, now branded as Attruby, for treating adults with transthyretin amyloidosis cardiomyopathy (ATTR-CM). Based on positive results from the ATTRibute-CM study, this approval has led to a surge in BBIO stock. The FDA’s endorsement of Attruby as the first product with a label specifying near-complete stabilization of TTR and its cardiovascular benefits makes the treatment a potential blockbuster.
The company plans to price Attruby at approximately $19k for a 28-day supply, which undercuts rival Pfizer’s Vyndamax. This move could potentially unsettle Pfizer, who achieved $3.3 billion in global revenue from its Vyndaqel franchise in 2023.
The approval also triggers a $500 million milestone payment to BridgeBio, eliminating a significant regulatory and funding hurdle.
BridgeBio’s Recent Financial Results
The company recently released results for Q3 2024. Revenue of $2.73 million was $0.65 million short of analysts’ expectations. Year-to-date revenue, however, increased by $208.4 million, primarily due to upfront payments and service revenue under the same agreements.
Operating costs and expenses for the quarter were $194.5 million, up $32.7 million, primarily due to increased SG&A expenses. The total increase for the nine months was $145.5 million, with notable increases in SG&A, R&D, and restructuring costs. Restructuring, impairment, and related charges were $4.6 million for Q3 and $10.9 million for the nine months.
The net loss attributable to BridgeBio’s common stockholders for the quarter was $162.0 million, translating to a GAAP EPS of -$0.86, which beat consensus estimates by $0.14.
The company’s cash, cash equivalents, and short-term restricted cash increased by $13.1 million to $405.7 million, with several contributing transactions.
Is BBIO a Buy?
As a start-up venture, the stock has had little in the way of predictable cash flows upon which to build a DCF model for valuation; thus, it has traded mainly based on investor sentiment. Over the past year, the stock has been volatile, declining by 15%. It trades at the lower end of its 52-week price range of $21.62 – $44.32, though the recent spike in price shows positive price momentum as it now trades above the 20-day (25.15) and 50-day (25.24) moving averages.
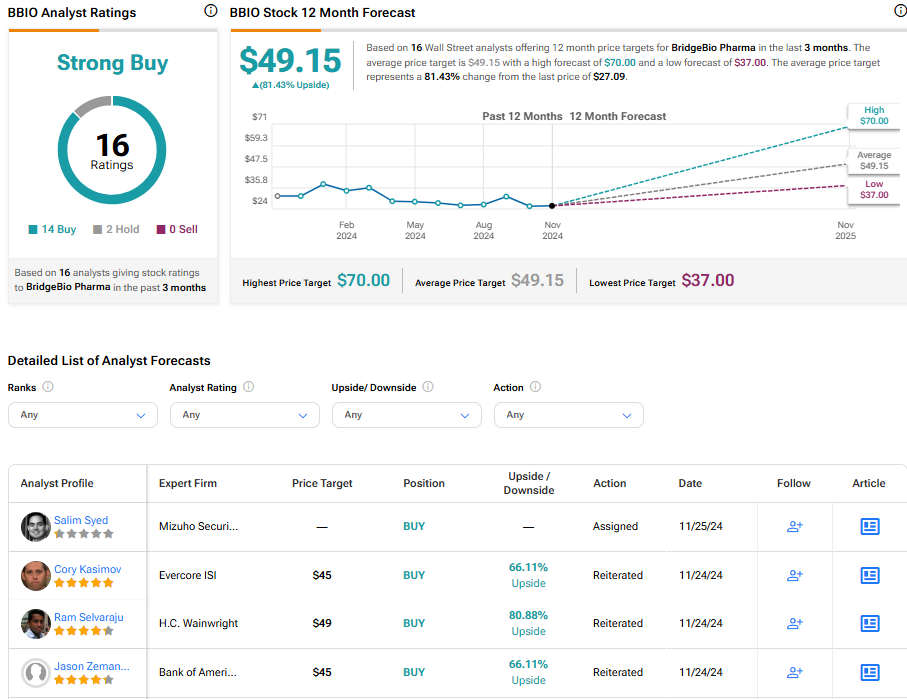
Analysts following the company have been bullish on its prospects. For instance, Scotiabank analyst Greg Harrison has recently increased the price target on the shares to $48, up from $45, maintaining an Outperform rating. He declared the recent approval of the TTR stabilizer acoramidis, a major victory for the company as it now enters the commercial stage with its debut product.
Bottom Line on BBIO
BridgeBio Pharma has realized a significant achievement with the FDA’s approval of Attruby, a treatment for adults with transthyretin amyloidosis cardiomyopathy (ATTR-CM). This approval is a game-changer for the company, propelling it into a stage of commercial maturity. These developments make the stock appealing to high-risk, high-reward biopharma industry investors.
















