Biotechnology company bluebird bio, Inc. (BLUE) announced that the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has approved the resumption of the marketing of medicinal products containing ZYNTEGLO, which is used to treat β- thalassemia.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Following the news release, shares of the company gained marginally to close at $30.57 in Friday’s trading session.
Although no instances of hematologic malignancy were reported in patients receiving treatment with ZYNTEGLO, the company decided to temporarily put on hold the marketing of ZYNTEGLO as it used the same BB305 lentiviral vector used in LentiGlobin for sickle cell disease.
The President of Severe Genetic Diseases at bluebird bio, Andrew Obenshain, said, “We are pleased to resume offering ZYNTEGLO to patients living with transfusion-dependent β-thalassemia, offering the potential to live free from transfusions, which is evidenced by our clinical studies where patients are maintaining normal or near-normal hemoglobin levels over the course of up to seven years of follow-up.” (See bluebird bio stock chart on TipRanks)
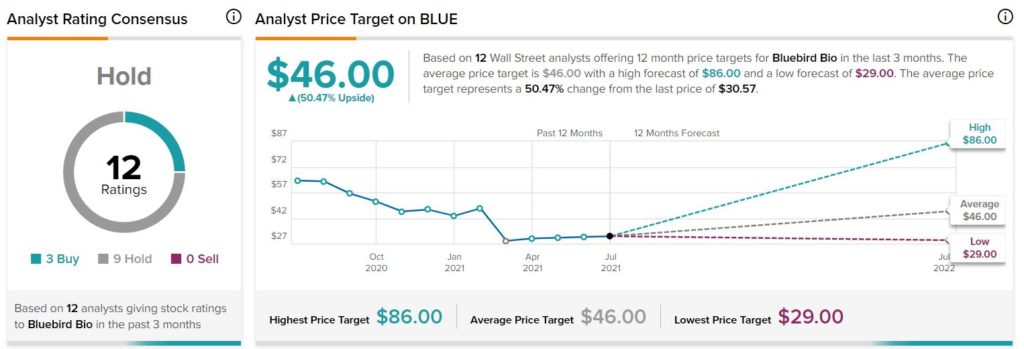
Recently, Berenberg Bank analyst Zhiqiang Shu initiated a Hold rating on the stock with a price target of $35. The analyst’s price target implies upside potential of 14.5% from current levels.
Consensus among analysts is a Hold based on 3 Buys and 9 Holds. The average bluebird bio price target stands at $46 (50.5% upside potential). Shares of the company have declined 49.7% over the past year.
Related News:
Brunswick Corporation’s Freedom Acquires Spain’s Fanautic Club
Bentley Systems’ Seequent Snaps up Aarhus GeoSoftware
Cisco Concludes Acquisition of Socio Labs









