BioNTech and Shanghai Fosun Pharmaceutical announced an agreement for the supply of an initial 100 million doses of their COVID-19 vaccine BNT162 to mainland China in 2021. BioNTech shares fell 2.8% in Thursday’s pre-market trading.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
BioNTech’s (BNTX) production facilities in Germany will distribute the initial supply of the vaccine, the company said. BioNTech is an immunotherapy company specializing in innovative therapies for cancer and other serious diseases. Shanghai Fosun Pharmaceutical (SFOSF) is a healthcare group in China.
The supply deal comes after the two companies entered into a strategic collaboration on Mar. 20 to target the development and commercialization of an experimental COVID-19 vaccine based on BioNTech’s mRNA technology platform.
They initiated the Phase 2 trial of the COVID-19 vaccine candidate in China, on Nov. 20, with 960 participants, aged between 18 and 85.
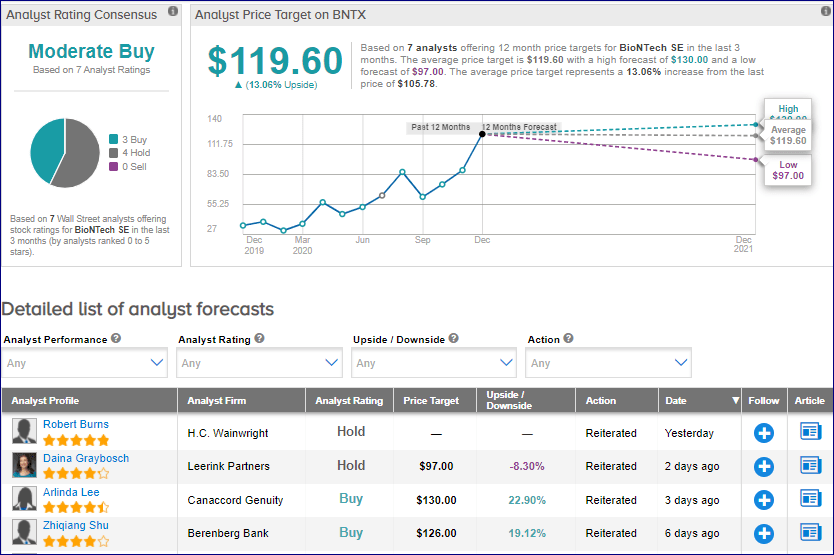
BNTX shares have gained a stellar 212.2% YTD and are trading at a discount of 19.3% to its 52-week high. (See BNTX stock analysis on TipRanks)
H.C. Wainwright analyst Robert Burns this week reiterated a Hold rating on the stock, citing the “rapidly evolving nature of the pandemic and the increasingly competitive COVID-19 vaccine arena.”
Burns believes that there is a “high probability” for the approval of BNT162b2 in Europe following the recent approval by the US Food and Drug Administration (FDA).
From the rest of the Street, the stock scores a cautiously optimistic Moderate Buy analyst consensus based on 3 Buys and 4 Holds. The average price target of $119.60 implies an upside of 13.1% to current levels.
Related News:
Anthem Appoints New Chief Health Officer; Street Is Bullish
Abbott Wins FDA Approval For At-Home Covid-19 Test; Street Says Buy
Amgen Declares 10% Dividend Hike; Street Expects Shares To Recover









