Shares of Biogen exploded 44% after staff at the US Food and Drug Administration (FDA) concluded in a review that there was “substantial evidence” that aducanumab, its investigational treatment for Alzheimer’s disease, was effective.
The stock jumped to $355.63 after FDA scientists said that the 302 Phase 3 study showed that the effect of aducanumab, developed by Biogen (BIIB) and Japan’s Eisai, is “robust and exceptionally persuasive”. The results of the study are appropriately viewed as supportive evidence of the effectiveness of aducanumab, the FDA added.
Back in July, Biogen submitted to the FDA a Biologics License Application (BLA) for approval to market aducanumab. If approved, aducanumab would become the first therapy to reduce the clinical decline of Alzheimer’s disease and would also be the first therapy to demonstrate that removing amyloid beta resulted in better clinical outcomes. Aducanumab is a human monoclonal antibody designed to treat early Alzheimer’s disease.
“Aducanumab, when dosed at 10 mg/kg IV every 4 weeks after a titration period of 24 weeks, has an acceptable safety profile that would support use in individuals with Alzheimer’s disease,” FDA staff said. “Based on the considerations above, the applicant has provided substantial evidence of effectiveness to support approval.”
The FDA review was published ahead of a panel of independent experts who are expected to meet on Friday to evaluate the recommendation of the drug’s approval to the FDA. A final call on the drug’s approval by the US regulator is due by March 2021.
In the US, more than 5.8 million people are living with Alzheimer’s disease. By 2050, this number is projected to more than double. Alzheimer’s disease was the sixth leading cause of death in the US and the fifth leading cause for people aged 65 years and older in 2018.
For now, J. P. Morgan analyst Cory Kasimov maintains a Hold rating on the stock with a $269 price target (24% downside potential), saying that the overarching takeaway from the review is that the FDA showed its cards, and it’s quite evident that the agency wants to approve the treatment.
“That said, our more thorough review was eye opening in terms of the conflict of opinions between the FDA reviewers and their statisticians, who are far more negative,” Kasimov wrote in a note to investors. “We doubt whether this makes a difference on Friday.” (See BIIB stock analysis on TipRanks)
“We suspect that even a close negative vote isn’t enough to dissuade the FDA on approval,” the analyst summed up.
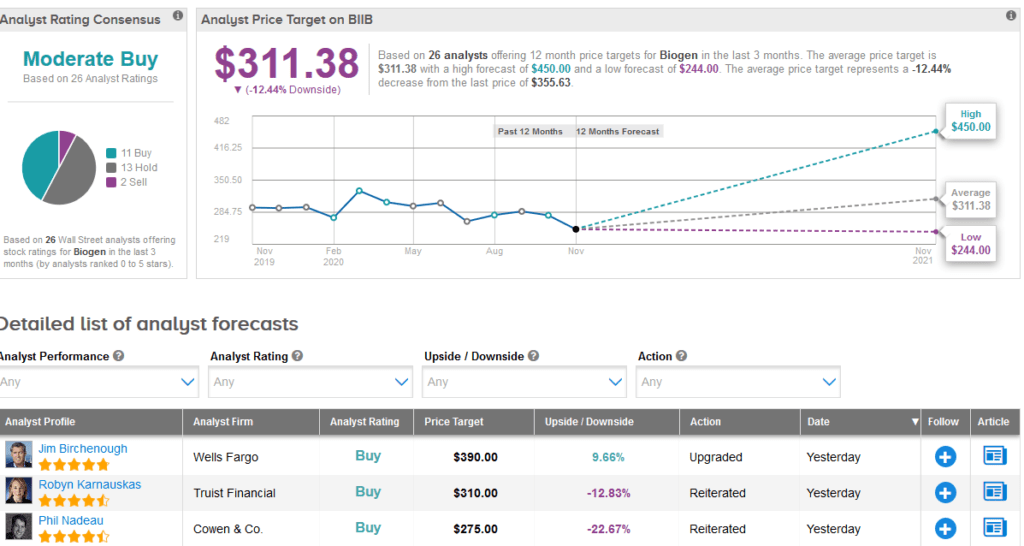
Meanwhile, the rest of the Street is cautiously optimistic on the stock. The Moderate Buy analyst consensus shows 11 Buys versus 13 Holds and 2 Sells. With shares up 20% so far this year, the $311.38 average price target implies 12% downside potential to current levels.
Related News:
J&J Strikes Covid-19 Vaccine Manufacturing Deal With Aspen
GWPH Explodes 21% As Epidiolex Roars Back Through The Pandemic
Opko Health Tanks 10% Despite Solid Q3 Covid Testing Revenue
















