Two Congressional Committees have raised concerns over the approval of Biogen’s (NASDAQ:BIIB) Alzheimer’s disease drug, Aduhelm, by the U.S. Food and Drug Administration (FDA). Also, both parties are charged with maintaining inaccurate records of the meetings and interactions between their representatives.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
After an 18-month investigation into its regulatory review and approval, the Committees discovered that “FDA’s review and approval of Aduhelm consisted of atypical procedures and deviated from the agency’s own guidance.”
Furthermore, the FDA granted accelerated approval for Aduhelm despite knowing that Biogen had stopped Aduhelm clinical trials in March 2019. The cancellation was based on an independent report that claimed the drug was less likely to slow cognitive and functional impairment.
The report also mentions that the FDA approved the drug even though its advisory panel did not vote in its favor and experts raised concerns about “the inconsistency of the drug’s clinical data.”
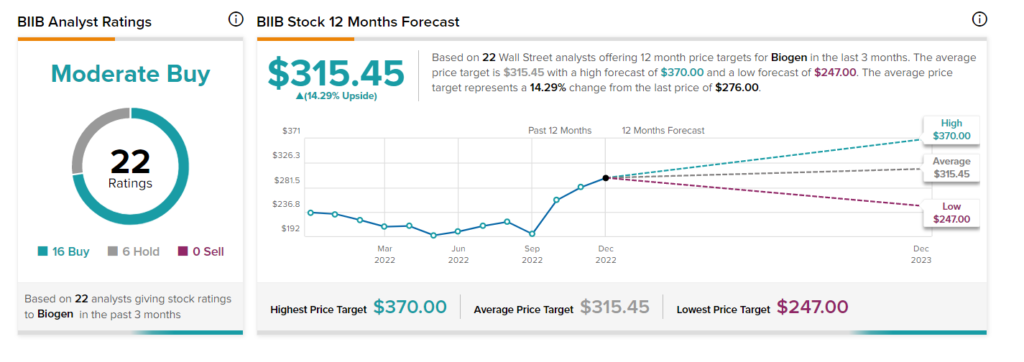
Is BIIB a Buy Stock?
The BIIB stock commands a Moderate Buy consensus rating based on 16 Buys and six Holds. The average Biogen stock price forecast of $315.45 implies 14.3% upside potential. Over the past three months, the stock has gained more than 3%.
Special end-of-year offer: Access TipRanks Premium tools for an all-time low price! Click to learn more.Disclosure









